The Mechanobiology of Aging
- PMID: 26643020
- PMCID: PMC4886230
- DOI: 10.1146/annurev-bioeng-071114-040829
The Mechanobiology of Aging
Abstract
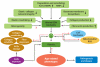
Aging is a complex, multifaceted process that induces a myriad of physiological changes over an extended period of time. Aging is accompanied by major biochemical and biomechanical changes at macroscopic and microscopic length scales that affect not only tissues and organs but also cells and subcellular organelles. These changes include transcriptional and epigenetic modifications; changes in energy production within mitochondria; and alterations in the overall mechanics of cells, their nuclei, and their surrounding extracellular matrix. In addition, aging influences the ability of cells to sense changes in extracellular-matrix compliance (mechanosensation) and to transduce these changes into biochemical signals (mechanotransduction). Moreover, following a complex positive-feedback loop, aging is accompanied by changes in the composition and structure of the extracellular matrix, resulting in changes in the mechanics of connective tissues in older individuals. Consequently, these progressive dysfunctions facilitate many human pathologies and deficits that are associated with aging, including cardiovascular, musculoskeletal, and neurodegenerative disorders and diseases. Here, we critically review recent work highlighting some of the primary biophysical changes occurring in cells and tissues that accompany the aging process.
Keywords: cellular mechanics; extracellular matrix; mitochondrial dysfunction; nuclear mechanics.
Figures



References
-
- Minimas DA. Ageing and its influence on wound healing. Wounds UK. 2007;3:42–50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

