Triage of HPV positive women in cervical cancer screening
- PMID: 26643050
- PMCID: PMC4789103
- DOI: 10.1016/j.jcv.2015.11.015
Triage of HPV positive women in cervical cancer screening
Abstract
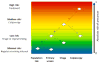
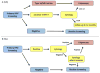
Despite HPV vaccines, screening will remain central for decades to control cervical cancer. Recently, HPV testing alone or with cytology was introduced as an alternative to cytology screening. However, most HPV infections are harmless and additional tests are required to identify women with progressing infections or precancer. With three options for primary screening, and without clear strategies for triage of screen-positive women, there is great confusion about the best approach. Also, increasing HPV vaccination coverage will lead to lower disease prevalence, and force new screening approaches. Currently recommended triage strategies for primary HPV screening include HPV genotyping for HPV16 and HPV18 and cytology. Other alternatives that are currently evaluated include p16/Ki-67 dual stain cytology, host methylation, and viral methylation testing. Clinical management of women with cervical cancer screening results is moving to use risk thresholds rather than individual test results. Specific risk thresholds have been defined for return to primary screening, repeat testing, referral to colposcopy, and immediate treatment. Choice of test algorithms is based on comparison of absolute risk estimates from triage tests with established clinical thresholds. Importantly, triage tests need to be evaluated together with the primary screening test and the downstream clinical management. An optimal integrated screening and triage strategy should reassure the vast majority of women that they are at very low risk of cervical cancer, send the women at highest risk to colposcopy at the right time, when disease can be colposcopically detected, and minimize the intermediate risk group that requires continued surveillance.
Keywords: Cervical cancer screening; Cytology; HPV; Methylation; Triage; p16/Ki-67.
Published by Elsevier B.V.
Conflict of interest statement
NCI has received cervical cancer screening assays in-kind or at reduced cost from BD, Cepheid, Hologic, and Roche.
Figures
References
-
- Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. European journal of cancer (Oxford, England: 1990) 2009;45:2640–8. - PubMed
-
- Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet (London, England) 1987;1:1247–9. - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet (London, England) 2007;370:890–907. - PubMed
-
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. The Lancet Oncology. 2008;9:425–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

