Towards Rational Dosing Algorithms for Vancomycin in Neonates and Infants Based on Population Pharmacokinetic Modeling
- PMID: 26643337
- PMCID: PMC4750654
- DOI: 10.1128/AAC.01968-15
Towards Rational Dosing Algorithms for Vancomycin in Neonates and Infants Based on Population Pharmacokinetic Modeling
Abstract
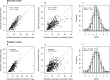
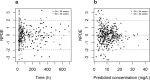
Because of the recent awareness that vancomycin doses should aim to meet a target area under the concentration-time curve (AUC) instead of trough concentrations, more aggressive dosing regimens are warranted also in the pediatric population. In this study, both neonatal and pediatric pharmacokinetic models for vancomycin were externally evaluated and subsequently used to derive model-based dosing algorithms for neonates, infants, and children. For the external validation, predictions from previously published pharmacokinetic models were compared to new data. Simulations were performed in order to evaluate current dosing regimens and to propose a model-based dosing algorithm. The AUC/MIC over 24 h (AUC24/MIC) was evaluated for all investigated dosing schedules (target of >400), without any concentration exceeding 40 mg/liter. Both the neonatal and pediatric models of vancomycin performed well in the external data sets, resulting in concentrations that were predicted correctly and without bias. For neonates, a dosing algorithm based on body weight at birth and postnatal age is proposed, with daily doses divided over three to four doses. For infants aged <1 year, doses between 32 and 60 mg/kg/day over four doses are proposed, while above 1 year of age, 60 mg/kg/day seems appropriate. As the time to reach steady-state concentrations varies from 155 h in preterm infants to 36 h in children aged >1 year, an initial loading dose is proposed. Based on the externally validated neonatal and pediatric vancomycin models, novel dosing algorithms are proposed for neonates and children aged <1 year. For children aged 1 year and older, the currently advised maintenance dose of 60 mg/kg/day seems appropriate.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

