Population Pharmacokinetics of Rifampin in Pregnant Women with Tuberculosis and HIV Coinfection in Soweto, South Africa
- PMID: 26643345
- PMCID: PMC4776013
- DOI: 10.1128/AAC.02051-15
Population Pharmacokinetics of Rifampin in Pregnant Women with Tuberculosis and HIV Coinfection in Soweto, South Africa
Abstract
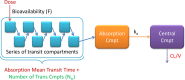
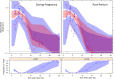
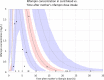
Effective treatment of tuberculosis during pregnancy is essential for preventing maternal and fetal mortality, but little is known about the effects of pregnancy on the disposition of antituberculosis drugs. We explored the effects of pregnancy on the pharmacokinetics of rifampin, the key sterilizing drug in tuberculosis treatment, in Tshepiso, a prospective cohort study involving pregnant HIV-infected women with or without tuberculosis in Soweto, South Africa. Participants receiving standard first-line tuberculosis treatment underwent sparse sampling for rifampin at 37 weeks' gestation or delivery and then postpartum. Cord blood was collected when possible. A population pharmacokinetic model was developed to investigate the effects of pregnancy on rifampin pharmacokinetics. Among the 48 participants, median age and weight were 28 years and 67 kg, respectively. A one-compartment model with first-order elimination, transit compartment absorption, and allometric scaling described the data well. Pregnancy reduced rifampin clearance by 14%. The median (interquartile range) model-estimated rifampin area under the concentration-time curve over 24 h (AUC0-24) during pregnancy or intrapartum was 40.8 h · mg/liter (27.1 to 54.2 h · mg/liter) compared to 37.4 h · mg/liter (26.8 to 50.3 h · mg/liter) postpartum. The maximum concentrations were similar during pregnancy and postpartum. Rifampin was detectable in 36% (8/22) of cord blood samples, and 88% (42/48) of the women had successful treatment outcomes. There was one case of perinatal tuberculosis. In conclusion, rifampin clearance is modestly reduced during the last trimester of pregnancy. Exposures are only slightly increased, so dose adjustment during pregnancy is not needed. Rifampin was detected in cord blood samples when delivery occurred soon after dosing. The consequences of exposure to this potent inducer of metabolizing enzymes among HIV-exposed infants are unclear.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- World Health Organization. 2014. Global tuberculosis report 2014. WHO/HTM/TB/2014.08. World Health Organization, Geneva, Switzerland.
-
- Savic RM, Weiner M, Kenzie MW, Helig C, Dooley K, Engle M, Nsubuga P, Phan H, Peloquin C, Dorman S, Tuberculosis Trials Consortium of the Centers for Disease Control and Prevention. 2013. PK/PD analysis of rifapentine in patients during intensive phase treatment for tuberculosis from Tuberculosis Trials Consortium studies 29 and 29X, abstr 15. Abstr 6th Int Workshop Clin Pharmacol Antituberc Drugs, Denver, CO, 9 September 2013.
-
- Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. - DOI - PMC - PubMed
-
- Ruslami R, Ganiem A, Dian RS, Apriani L, Achmad TH, van der Ven AJ, Borm G, Aarnoutse RE, Van Crevel R. 2013. Intensified regimen containing rifampin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 13:27–35. doi: 10.1016/S1473-3099(12)70264-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

