Research START: A Multimethod Study of Barriers and Accelerators of Recruiting Research Participants
- PMID: 26643413
- PMCID: PMC4753776
- DOI: 10.1111/cts.12351
Research START: A Multimethod Study of Barriers and Accelerators of Recruiting Research Participants
Abstract
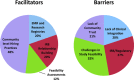
Under-recruitment into clinical trials is a common and costly problem that undermines medical research. To better understand barriers to recruitment into clinical trials in our region, we conducted a multimethod descriptive study. We initially surveyed investigators who had conducted or were currently conducting studies that utilized an adult or pediatric clinical research center (n = 92). We then conducted focus groups and key informant interviews with investigators, coordinators, and other stakeholders in clinical and translational research (n = 32 individuals). Only 41% of respondents reported that they had or were successfully meeting recruitment goals and 24% of the closed studies actually met their targeted recruitment goals. Varied reasons were identified for poor recruitment but there was not a single investigator or study "phenotype" that predicted enrollment outcome. Investigators commonly recruited from their own practice or clinic, and 29% used a manual electronic medical record search. The majority of investigators would utilize a service that provides recruitment advice, including feasibility assessment and consultation, easier access to the electronic health record and assistance with institutional review board and other regulatory requirements. Our findings suggest potential benefits providing assistance across a range of services that can be individualized to the varied needs of clinical and translational investigators.
Keywords: participant recruitment; registries; regulatory science.
© 2015 Wiley Periodicals, Inc.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous