Cutting Edge: IL-1 Receptor Signaling is Critical for the Development of Autoimmune Uveitis
- PMID: 26643477
- PMCID: PMC4707108
- DOI: 10.4049/jimmunol.1502080
Cutting Edge: IL-1 Receptor Signaling is Critical for the Development of Autoimmune Uveitis
Abstract
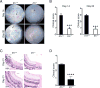
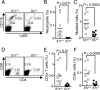
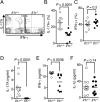
IL-1β is a proinflammatory cytokine important for local and systemic immunity. However, aberrant production of this cytokine is implicated in pathogenic mechanisms of a number of inflammatory diseases, including Behçet's disease and age-related macular degeneration. In this study, we report the increased secretion of IL-1β in the retina by neutrophils, macrophages, and dendritic cells during ocular inflammation and show that loss of IL-1R signaling confers protection from experimental autoimmune uveitis. Moreover, the amelioration of experimental autoimmune uveitis in Il1r-deficient mice was associated with reduced infiltration of inflammatory cells into the retina and decreased numbers of uveitogenic Th17 cells that mediate uveitis. These findings indicate the possible utility of IL-1R-blocking agents for the treatment of ocular inflammatory diseases.
Figures

References
-
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. - PubMed
-
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends in immunology. 2011;32:110–116. - PubMed
-
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature reviews Rheumatology. 2010;6:232–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

