Cardiovascular disease following hematopoietic stem cell transplantation: Pathogenesis, detection, and the cardioprotective role of aerobic training
- PMID: 26643524
- PMCID: PMC5003053
- DOI: 10.1016/j.critrevonc.2015.11.007
Cardiovascular disease following hematopoietic stem cell transplantation: Pathogenesis, detection, and the cardioprotective role of aerobic training
Abstract
Advances in hematopoietic cell transplantation (HCT) techniques and supportive care strategies have led to dramatic improvements in relapse mortality in patients with high-risk hematological malignancies. These improvements, however, conversely increase the risk of late-occurring non-cancer competing causes, mostly cardiovascular disease (CVD). HCT recipients have a significantly increased risk of CVD-specific mortality, including elevated incidence of coronary artery disease (CAD), cerebrovascular disease, and heart failure (HF) compared to age-matched counterparts. Accordingly, there is an urgent need to identify techniques for the detection of early CVD in HCT patients to inform early prevention strategies. Aerobic training (AT) is established as the cornerstone of primary and secondary disease prevention in multiple clinical settings, and may confer similar benefits in HCT patients at high-risk of CVD. The potential benefits of AT either before, immediately after, or in the months/years following HCT have received limited attention. Here, we discuss the risk and extent of CVD in adult HCT patients, highlight novel tools for early detection of CVD, and review existing evidence in oncology and non-oncology populations supporting the efficacy of AT to attenuate HCT-induced CVD. This knowledge can be utilized to optimize treatment, while minimizing CVD risk in individuals with hematological malignancies undergoing HCT.
Keywords: Cardiovascular disease; Detection; Exercise; Hematopoietic stem cell transplantation.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
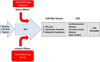
Figures


References
-
- Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. - PubMed
-
- Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone marrow transplantation. 2012;47(5):619–625. - PubMed
-
- Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

