SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment
- PMID: 26643973
- PMCID: PMC4686651
- DOI: 10.1038/ncomms10004
SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment
Abstract
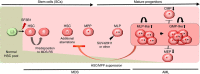
Despite the recent evidence of the existence of myelodysplastic syndrome (MDS) stem cells in 5q-MDS patients, it is unclear whether haematopoietic stem cells (HSCs) could also be the initiating cells in other MDS subgroups. Here we demonstrate that SF3B1 mutation(s) in our cohort of MDS patients with ring sideroblasts can arise from CD34(+)CD38(-)CD45RA(-)CD90(+)CD49f(+) HSCs and is an initiating event in disease pathogenesis. Xenotransplantation of SF3B1 mutant HSCs leads to persistent long-term engraftment restricted to myeloid lineage. Moreover, genetically diverse evolving subclones of mutant SF3B1 exist in mice, indicating a branching multi-clonal as well as ancestral evolutionary paradigm. Subclonal evolution in mice is also seen in the clinical evolution in patients. Sequential sample analysis shows clonal evolution and selection of the malignant driving clone leading to AML transformation. In conclusion, our data show SF3B1 mutations can propagate from HSCs to myeloid progeny, therefore providing a therapeutic target.
Figures





References
-
- Mufti G. J. et al. Diagnosis and classification of myelodysplastic syndrome: International Working Group on Morphology of myelodysplastic syndrome (IWGM-MDS) consensus proposals for the definition and enumeration of myeloblasts and ring sideroblasts. Haematologica 93, 1712–1717 (2008). - PubMed
-
- Tefferi A. & Vardiman J. W. Myelodysplastic syndromes. N. Engl. J. Med. 361, 1872–1885 (2009). - PubMed
-
- Yoshida K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

