Hypoxia facilitates neurogenic dural plasma protein extravasation in mice: a novel animal model for migraine pathophysiology
- PMID: 26644235
- PMCID: PMC4672320
- DOI: 10.1038/srep17845
Hypoxia facilitates neurogenic dural plasma protein extravasation in mice: a novel animal model for migraine pathophysiology
Abstract
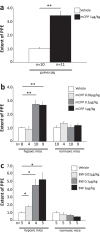
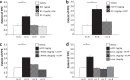
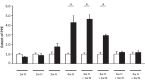
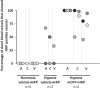
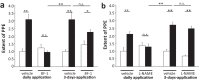
Migraine animal models generally mimic the onset of attacks and acute treatment processes. A guinea pig model used the application of meta-chlorophenylpiperazine (mCPP) to trigger immediate dural plasma protein extravasation (PPE) mediated by 5-HT2B receptors. This model has predictive value for antimigraine drugs but cannot explain the delayed onset of efficacy of 5-HT2B receptor antagonists when clinically used for migraine prophylaxis. We found that mCPP failed to induce dural PPE in mice. Considering the role 5-HT2B receptors play in hypoxia-induced pulmonary vessel muscularization, we were encouraged to keep mice under hypoxic conditions and tested whether this treatment will render them susceptible to mCPP-induced dural PPE. Following four-week of hypoxia, PPE, associated with increased transendothelial transport, was induced by mCPP. The effect was blocked by sumatriptan. Chronic application of 5-HT2B receptor or nitric oxide synthase blockers during hypoxia prevented the development of susceptibility. Here we present a migraine model that distinguishes between a migraine-like state (hypoxic mice) and normal, normoxic mice and mimics processes that are related to chronic activation of 5-HT2B receptors under hypoxia. It seems striking, that chronic endogenous activation of 5-HT2B receptors is crucial for the sensitization since 5-HT2B receptor antagonists have strong, albeit delayed migraine prophylactic efficacy.
Figures


References
-
- Bergerot A. et al. Animal models of migraine: looking at the component parts of a complex disorder. Eur J Neurosci 24, 1517–1534 (2006). - PubMed
-
- Eikermann-Haerter K. & Moskowitz M. A. Animal models of migraine headache and aura. Curr Opin Neurol 21, 294–300 (2008). - PubMed
-
- Messlinger K. Migraine: where and how does the pain originate? Exp Brain Res 196, 179–193 (2009). - PubMed
-
- Yu X. J. et al. 5-Carboxamido-tryptamine, CP-122,288 and dihydroergotamine but not sumatriptan, CP-93,129, and serotonin-5-O-carboxymethyl-glycyl -tyrosinamide block dural plasma protein extravasation in knockout mice that lack 5-hydroxytryptamine1B receptors. Mol Pharmacol 49, 761–765 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

