CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus japonicus
- PMID: 26644503
- PMCID: PMC4734552
- DOI: 10.1104/pp.15.00650
CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus japonicus
Abstract
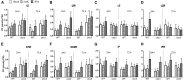
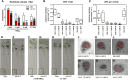
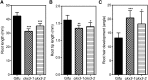
Cytokinins are required for symbiotic nodule development in legumes, and cytokinin signaling responses occur locally in nodule primordia and in developing nodules. Here, we show that the Lotus japonicus Ckx3 cytokinin oxidase/dehydrogenase gene is induced by Nod factor during the early phase of nodule initiation. At the cellular level, pCkx3::YFP reporter-gene studies revealed that the Ckx3 promoter is active during the first cortical cell divisions of the nodule primordium and in growing nodules. Cytokinin measurements in ckx3 mutants confirmed that CKX3 activity negatively regulates root cytokinin levels. Particularly, tZ and DHZ type cytokinins in both inoculated and uninoculated roots were elevated in ckx3 mutants, suggesting that these are targets for degradation by the CKX3 cytokinin oxidase/dehydrogenase. The effect of CKX3 on the positive and negative roles of cytokinin in nodule development, infection and regulation was further clarified using ckx3 insertion mutants. Phenotypic analysis indicated that ckx3 mutants have reduced nodulation, infection thread formation and root growth. We also identify a role for cytokinin in regulating nodulation and nitrogen fixation in response to nitrate as ckx3 phenotypes are exaggerated at increased nitrate levels. Together, these findings show that cytokinin accumulation is tightly regulated during nodulation in order to balance the requirement for cell divisions with negative regulatory effects of cytokinin on infection events and root development.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures




Similar articles
-
Knockdown of LjIPT3 influences nodule development in Lotus japonicus.Plant Cell Physiol. 2014 Jan;55(1):183-93. doi: 10.1093/pcp/pct171. Epub 2013 Nov 26. Plant Cell Physiol. 2014. PMID: 24285753
-
Cytokinin Biosynthesis Promotes Cortical Cell Responses during Nodule Development.Plant Physiol. 2017 Sep;175(1):361-375. doi: 10.1104/pp.17.00832. Epub 2017 Jul 21. Plant Physiol. 2017. PMID: 28733389 Free PMC article.
-
A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis.Science. 2007 Jan 5;315(5808):101-4. doi: 10.1126/science.1132514. Epub 2006 Nov 16. Science. 2007. PMID: 17110535
-
Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria.Int Rev Cell Mol Biol. 2015;316:111-58. doi: 10.1016/bs.ircmb.2015.01.004. Epub 2015 Feb 20. Int Rev Cell Mol Biol. 2015. PMID: 25805123 Review.
-
Into the Root: How Cytokinin Controls Rhizobial Infection.Trends Plant Sci. 2016 Mar;21(3):178-186. doi: 10.1016/j.tplants.2015.09.003. Epub 2015 Oct 14. Trends Plant Sci. 2016. PMID: 26459665 Review.
Cited by
-
Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation.Plant Cell. 2020 Jan;32(1):15-41. doi: 10.1105/tpc.19.00279. Epub 2019 Oct 24. Plant Cell. 2020. PMID: 31649123 Free PMC article. Review.
-
Genes for asparagine metabolism in Lotus japonicus: differential expression and interconnection with photorespiration.BMC Genomics. 2017 Oct 12;18(1):781. doi: 10.1186/s12864-017-4200-x. BMC Genomics. 2017. PMID: 29025409 Free PMC article.
-
Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia.Nat Commun. 2017 Feb 23;8:14534. doi: 10.1038/ncomms14534. Nat Commun. 2017. PMID: 28230048 Free PMC article.
-
A Laser Dissection-RNAseq Analysis Highlights the Activation of Cytokinin Pathways by Nod Factors in the Medicago truncatula Root Epidermis.Plant Physiol. 2016 Jul;171(3):2256-76. doi: 10.1104/pp.16.00711. Epub 2016 May 23. Plant Physiol. 2016. PMID: 27217496 Free PMC article.
-
Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation.Plant Biotechnol J. 2020 Nov;18(11):2225-2240. doi: 10.1111/pbi.13378. Epub 2020 Sep 1. Plant Biotechnol J. 2020. PMID: 32181964 Free PMC article.
References
-
- Bauer P, Ratet P, Crespi MD, Schultze M, Kondorosi A (1996) Nod factors and cytokinins induce similar cortical cell division, amyloplast deposition and MsEnod12A expression patterns in alfalfa roots. Plant J 10: 91–105
-
- Breakspear A, Liu C, Roy S, Stacey N, Rogers C, Trick M, Morieri G, Mysore KS, Wen J, Oldroyd GED, Downie JA, Murray JD (2014) The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for Auxin signaling in rhizobial infection. Plant Cell 26: 4680–4701 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

