Increased Sucrose Accumulation Regulates Iron-Deficiency Responses by Promoting Auxin Signaling in Arabidopsis Plants
- PMID: 26644507
- PMCID: PMC4734570
- DOI: 10.1104/pp.15.01598
Increased Sucrose Accumulation Regulates Iron-Deficiency Responses by Promoting Auxin Signaling in Arabidopsis Plants
Abstract
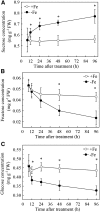
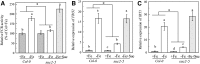
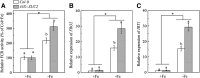
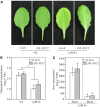
Previous studies have identified that auxins acts upstream of nitric oxide in regulating iron deficiency responses in roots, but the upstream signaling molecule of auxins remains unknown. In this study, we showed that Fe deficiency increased sucrose (Suc) level in roots of Arabidopsis (Arabidopsis thaliana). Exogenous application of Suc further stimulated Fe deficiency-induced ferric-chelate-reductase (FCR) activity and expression of Fe acquisition-related genes FRO2, IRT1, and FIT in roots. The opposite patterns were observed in the dark treatment. In addition, FCR activity and expression of Fe acquisition-related genes were higher in the Suc high-accumulating transgenic plant 35S::SUC2 but were lower in the Suc low-accumulating mutant suc2-5 compared with wild-type plants under Fe-deficient conditions. Consequently, Fe deficiency tolerance was enhanced in 35S::SUC2 but was compromised in suc2-5. Exogenous Suc also increased root β-glucuronidase (GUS) activity in auxin-inducible reporter DR5-GUS transgenic plants under Fe deficiency. However, exogenous Suc failed to increase FCR activity and expression of Fe acquisition-related genes in the auxin transport-impaired mutants aux1-7 and pin1-1 as well as in the wild-type plants treated with an auxin transport inhibitor under Fe deficiency. In summary, we found that increased Suc accumulation is required for regulating Fe deficiency responses in plants, with auxins acting downstream in transmitting the Fe deficiency signal.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures






Similar articles
-
Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis.Plant Physiol. 2010 Oct;154(2):810-9. doi: 10.1104/pp.110.161109. Epub 2010 Aug 10. Plant Physiol. 2010. PMID: 20699398 Free PMC article.
-
Proteasome-mediated turnover of the transcriptional activator FIT is required for plant iron-deficiency responses.Plant J. 2011 Jun;66(6):1044-52. doi: 10.1111/j.1365-313X.2011.04565.x. Epub 2011 Apr 5. Plant J. 2011. PMID: 21426424
-
Glutathione plays an essential role in nitric oxide-mediated iron-deficiency signaling and iron-deficiency tolerance in Arabidopsis.Plant J. 2015 Nov;84(3):464-77. doi: 10.1111/tpj.13011. Plant J. 2015. PMID: 26333047
-
Getting a sense for signals: regulation of the plant iron deficiency response.Biochim Biophys Acta. 2012 Sep;1823(9):1521-30. doi: 10.1016/j.bbamcr.2012.03.010. Epub 2012 Mar 28. Biochim Biophys Acta. 2012. PMID: 22483849 Free PMC article. Review.
-
FIT, a regulatory hub for iron deficiency and stress signaling in roots, and FIT-dependent and -independent gene signatures.J Exp Bot. 2020 Mar 12;71(5):1694-1705. doi: 10.1093/jxb/eraa012. J Exp Bot. 2020. PMID: 31922570 Free PMC article. Review.
Cited by
-
Concentration-dependent dual effects of exogenous sucrose on nitrogen metabolism in Andrographis paniculata.Sci Rep. 2022 Mar 22;12(1):4906. doi: 10.1038/s41598-022-08971-x. Sci Rep. 2022. PMID: 35318399 Free PMC article.
-
The phyB-dependent induction of HY5 promotes iron uptake by systemically activating FER expression.EMBO Rep. 2021 Jul 5;22(7):e51944. doi: 10.15252/embr.202051944. Epub 2021 May 20. EMBO Rep. 2021. PMID: 34018302 Free PMC article.
-
Metabolism of Malus halliana Roots Provides Insights into Iron Deficiency Tolerance Mechanisms.Plants (Basel). 2024 Sep 6;13(17):2500. doi: 10.3390/plants13172500. Plants (Basel). 2024. PMID: 39273984 Free PMC article.
-
Arabidopsis root apical meristem survival during waterlogging is determined by phytoglobin through nitric oxide and auxin.Planta. 2023 Sep 25;258(5):86. doi: 10.1007/s00425-023-04239-4. Planta. 2023. PMID: 37747517
-
Shortcutting Photorespiration Protects Potato Photosynthesis and Tuber Yield Against Heatwave Stress.Glob Chang Biol. 2024 Dec;30(12):e17595. doi: 10.1111/gcb.17595. Glob Chang Biol. 2024. PMID: 39629558 Free PMC article.
References
-
- Bacaicoa E, Mora V, Zamarreño ÁM, Fuentes M, Casanova E, García-Mina JM (2011) Auxin: a major player in the shoot-to-root regulation of root Fe-stress physiological responses to Fe deficiency in cucumber plants. Plant Physiol Biochem 49: 545–556 - PubMed
-
- Chandran D, Reinders A, Ward JM (2003) Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem 278: 44320–44325 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

