Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial
- PMID: 26644527
- PMCID: PMC4872028
- DOI: 10.1200/JCO.2015.63.0830
Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial
Erratum in
-
ERRATUM.J Clin Oncol. 2016 Jun 20;34(18):2198. doi: 10.1200/JCO.2016.68.4555. J Clin Oncol. 2016. PMID: 27281229 Free PMC article. No abstract available.
-
Errata.J Clin Oncol. 2019 Feb 20;37(6):528. doi: 10.1200/JCO.19.00057. J Clin Oncol. 2019. PMID: 30768919 Free PMC article. No abstract available.
Abstract
Purpose: There is growing interest to enhance symptom monitoring during routine cancer care using patient-reported outcomes, but evidence of impact on clinical outcomes is limited.
Methods: We randomly assigned patients receiving routine outpatient chemotherapy for advanced solid tumors at Memorial Sloan Kettering Cancer Center to report 12 common symptoms via tablet computers or to receive usual care consisting of symptom monitoring at the discretion of clinicians. Those with home computers received weekly e-mail prompts to report between visits. Treating physicians received symptom printouts at visits, and nurses received e-mail alerts when participants reported severe or worsening symptoms. The primary outcome was change in health-related quality of life (HRQL) at 6 months compared with baseline, measured by the EuroQol EQ-5D Index. Secondary endpoints included emergency room (ER) visits, hospitalizations, and survival.
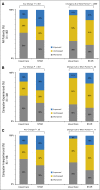
Results: Among 766 patients allocated, HRQL improved among more participants in the intervention group than usual care (34% v 18%) and worsened among fewer (38% v 53%; P < .001). Overall, mean HRQL declined by less in the intervention group than usual care (1.4- v 7.1-point drop; P < .001). Patients receiving intervention were less frequently admitted to the ER (34% v 41%; P = .02) or hospitalized (45% v 49%; P = .08) and remained on chemotherapy longer (mean, 8.2 v 6.3 months; P = .002). Although 75% of the intervention group was alive at 1 year, 69% with usual care survived the year (P = .05), with differences also seen in quality-adjusted survival (mean of 8.7 v. 8.0 months; P = .004). Benefits were greater for participants lacking prior computer experience. Most patients receiving intervention (63%) reported severe symptoms during the study. Nurses frequently initiated clinical actions in response to e-mail alerts.
Conclusion: Clinical benefits were associated with symptom self-reporting during cancer care.
Trial registration: ClinicalTrials.gov NCT00578006.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures

Comment in
-
PRO-cision Medicine: Personalizing Patient Care Using Patient-Reported Outcomes.J Clin Oncol. 2016 Feb 20;34(6):527-9. doi: 10.1200/JCO.2015.64.9491. Epub 2015 Dec 7. J Clin Oncol. 2016. PMID: 26644538 No abstract available.
-
Symptom Improvement Requires More Than Screening and Feedback.J Clin Oncol. 2016 Sep 20;34(27):3351-2. doi: 10.1200/JCO.2016.67.7708. Epub 2016 Jul 18. J Clin Oncol. 2016. PMID: 27432929 No abstract available.
-
Reply to K. Kroenke et al.J Clin Oncol. 2016 Sep 20;34(27):3352. doi: 10.1200/JCO.2016.67.9480. Epub 2016 Jul 18. J Clin Oncol. 2016. PMID: 27432931 No abstract available.
References
-
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer 2008;16:791-801. - PubMed
-
- Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

