Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice
- PMID: 26645545
- PMCID: PMC4946956
- DOI: 10.1097/j.pain.0000000000000439
Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice
Abstract
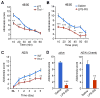
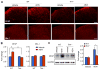
Increasing evidence suggests that Toll-like receptor 4 (TLR4) contributes importantly to spinal cord glial activation and chronic pain sensitization; however, its unique role in acute and chronic itch is unclear. In this study, we investigated the involvement of TLR4 in acute and chronic itch models in male mice using both transgenic and pharmacological approaches. Tlr4 mice exhibited normal acute itch induced by compound 48/80 and chloroquine, but these mice showed substantial reductions in scratching in chronic itch models of dry skin, induced by acetone and diethylether followed by water (AEW), contact dermatitis, and allergic contact dermatitis on the neck. Intrathecal (spinal) inhibition of TLR4 with lipopolysaccharide Rhodobacter sphaeroides did not affect acute itch but suppressed AEW-induced chronic itch. Compound 48/80 and AEW also produced robust alloknesis, a touch-elicited itch in wild-type mice, which was suppressed by intrathecal lipopolysaccharide R sphaeroides and Tlr4 deletion. Acetone and diethylether followed by water induced persistent upregulation of Tlr4 mRNA and increased TLR4 expression in GFAP-expressing astrocytes in spinal cord dorsal horn. Acetone and diethylether followed by water also induced TLR4-dependent astrogliosis (GFAP upregulation) in spinal cord. Intrathecal injection of astroglial inhibitor L-α-aminoadipate reduced AEW-induced chronic itch and alloknesis without affecting acute itch. Spinal TLR4 was also necessary for AEW-induced chronic itch in the cheek model. Interestingly, scratching plays an essential role in spinal astrogliosis because AEW-induced astrogliosis was abrogated by putting Elizabethan collars on the neck to prevent scratching the itchy skin. Our findings suggest that spinal TLR4 signaling is important for spinal astrocyte activation and astrogliosis that may underlie alloknesis and chronic itch.
Conflict of interest statement
All the authors have no competing financial interest in this study.
Figures






References
-
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155:1802–1813. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

