Association of Inhaled Corticosteroids with Incident Pneumonia and Mortality in COPD Patients; Systematic Review and Meta-Analysis
- PMID: 26645797
- PMCID: PMC4951104
- DOI: 10.3109/15412555.2015.1081162
Association of Inhaled Corticosteroids with Incident Pneumonia and Mortality in COPD Patients; Systematic Review and Meta-Analysis
Abstract
Background: Inhaled corticosteroids are commonly prescribed for patients with severe COPD. They have been associated with increased risk of pneumonia but not with increased pneumonia-associated or overall mortality.
Methods: To further examine the effects of inhaled corticosteroids on pneumonia incidence, and mortality in COPD patients, we searched for potentially relevant articles in PubMed, Medline, CENTRAL, EMBASE, Scopus, Web of Science and manufacturers' web clinical trial registries from 1994 to February 4, 2014. Additionally, we checked the included and excluded studies' bibliographies. We subsequently performed systematic review and meta-analysis of included randomized controlled trials and observational studies on the topic.
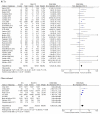
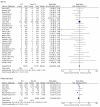
Results: We identified 38 studies: 29 randomized controlled trials and nine observational studies. The estimated unadjusted risk of pneumonia was increased in randomized trials: RR 1.61; 95% CI 1.35-1.93, p < 0.001; as well as in observational studies: OR 1.89; 95% CI 1.39-2.58, p < 0·001. Six randomized trials and seven observational studies were useful in estimating unadjusted risk of pneumonia -case-fatality: RR 0.91; 95% CI 0.52-1.59, p = 0.74; and OR 0.72; 95% CI 0.59-0.88, p = 0.001, respectively. Twenty-nine randomized trials and six observational studies allowed estimation of unadjusted risk of overall mortality: RR 0.95; 95% CI 0.85-1.05, p = 0.31; and OR 0.79; 95% CI 0.65-0.97, p = 0.02, respectively.
Conclusions: Despite a substantial and significant increase in unadjusted risk of pneumonia associated with inhaled corticosteroid use, pneumonia fatality and overall mortality were found not to be increased in randomized controlled trials and were decreased in observational studies.
Keywords: bias; case-fatality; drop-out; heterogeneity.
Figures


References
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 cited; Available from: Available at: http://www.goldcopd.org/ - PubMed
-
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. - PubMed
-
- Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 mug) or salmeterol (50 mug) on COPD exacerbations. Respir Med. 2008;102(8):1099–108. - PubMed
-
- Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–9. - PubMed
-
- Calverley PM, Stockley RA, Seemungal TA, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139(3):505–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
