Structural Insight into How Bacteria Prevent Interference between Multiple Divergent Type IV Secretion Systems
- PMID: 26646013
- PMCID: PMC4676284
- DOI: 10.1128/mBio.01867-15
Structural Insight into How Bacteria Prevent Interference between Multiple Divergent Type IV Secretion Systems
Abstract
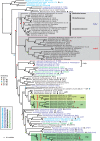
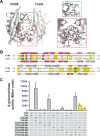
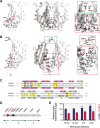
Prokaryotes use type IV secretion systems (T4SSs) to translocate substrates (e.g., nucleoprotein, DNA, and protein) and/or elaborate surface structures (i.e., pili or adhesins). Bacterial genomes may encode multiple T4SSs, e.g., there are three functionally divergent T4SSs in some Bartonella species (vir, vbh, and trw). In a unique case, most rickettsial species encode a T4SS (rvh) enriched with gene duplication. Within single genomes, the evolutionary and functional implications of cross-system interchangeability of analogous T4SS protein components remains poorly understood. To lend insight into cross-system interchangeability, we analyzed the VirB8 family of T4SS channel proteins. Crystal structures of three VirB8 and two TrwG Bartonella proteins revealed highly conserved C-terminal periplasmic domain folds and dimerization interfaces, despite tremendous sequence divergence. This implies remarkable structural constraints for VirB8 components in the assembly of a functional T4SS. VirB8/TrwG heterodimers, determined via bacterial two-hybrid assays and molecular modeling, indicate that differential expression of trw and vir systems is the likely barrier to VirB8-TrwG interchangeability. We also determined the crystal structure of Rickettsia typhi RvhB8-II and modeled its coexpressed divergent paralog RvhB8-I. Remarkably, while RvhB8-I dimerizes and is structurally similar to other VirB8 proteins, the RvhB8-II dimer interface deviates substantially from other VirB8 structures, potentially preventing RvhB8-I/RvhB8-II heterodimerization. For the rvh T4SS, the evolution of divergent VirB8 paralogs implies a functional diversification that is unknown in other T4SSs. Collectively, our data identify two different constraints (spatiotemporal for Bartonella trw and vir T4SSs and structural for rvh T4SSs) that mediate the functionality of multiple divergent T4SSs within a single bacterium.
Importance: Assembly of multiprotein complexes at the right time and at the right cellular location is a fundamentally important task for any organism. In this respect, bacteria that express multiple analogous type IV secretion systems (T4SSs), each composed of around 12 different components, face an overwhelming complexity. Our work here presents the first structural investigation on factors regulating the maintenance of multiple T4SSs within a single bacterium. The structural data imply that the T4SS-expressing bacteria rely on two strategies to prevent cross-system interchangeability: (i) tight temporal regulation of expression or (ii) rapid diversification of the T4SS components. T4SSs are ideal drug targets provided that no analogous counterparts are known from eukaryotes. Drugs targeting the barriers to cross-system interchangeability (i.e., regulators) could dysregulate the structural and functional independence of discrete systems, potentially creating interference that prevents their efficient coordination throughout bacterial infection.
Copyright © 2015 Gillespie et al.
Figures



Similar articles
-
The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion.Pathog Dis. 2016 Aug;74(6):ftw058. doi: 10.1093/femspd/ftw058. Epub 2016 Jun 14. Pathog Dis. 2016. PMID: 27307105 Free PMC article.
-
Structural Analysis and Inhibition of TraE from the pKM101 Type IV Secretion System.J Biol Chem. 2016 Nov 4;291(45):23817-23829. doi: 10.1074/jbc.M116.753327. Epub 2016 Sep 15. J Biol Chem. 2016. PMID: 27634044 Free PMC article.
-
Structure of the enterococcal T4SS protein PrgL reveals unique dimerization interface in the VirB8 protein family.Structure. 2022 Jun 2;30(6):876-885.e5. doi: 10.1016/j.str.2022.03.013. Epub 2022 Apr 15. Structure. 2022. PMID: 35429437
-
Biological and Structural Diversity of Type IV Secretion Systems.Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.psib-0012-2018. doi: 10.1128/microbiolspec.PSIB-0012-2018. Microbiol Spectr. 2019. PMID: 30953428 Free PMC article. Review.
-
Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction.Cell Microbiol. 2008 Aug;10(8):1591-8. doi: 10.1111/j.1462-5822.2008.01171.x. Epub 2008 Jul 30. Cell Microbiol. 2008. PMID: 18489724 Free PMC article. Review.
Cited by
-
Improved algorithms for quantifying the near symmetry of proteins: complete side chains analysis.J Cheminform. 2019 Jun 6;11(1):39. doi: 10.1186/s13321-019-0360-9. J Cheminform. 2019. PMID: 31172379 Free PMC article.
-
Salmonella genomic island 1 (SGI1) reshapes the mating apparatus of IncC conjugative plasmids to promote self-propagation.PLoS Genet. 2017 Mar 29;13(3):e1006705. doi: 10.1371/journal.pgen.1006705. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28355215 Free PMC article.
-
Transcriptional Response of Wolbachia to Dengue Virus Infection in Cells of the Mosquito Aedes aegypti.mSphere. 2021 Jun 30;6(3):101128msphere0043321. doi: 10.1128/mSphere.00433-21. Epub 2021 Jun 30. mSphere. 2021. PMID: 34190587 Free PMC article.
-
Evaluation of changes to the Rickettsia rickettsii transcriptome during mammalian infection.PLoS One. 2017 Aug 23;12(8):e0182290. doi: 10.1371/journal.pone.0182290. eCollection 2017. PLoS One. 2017. PMID: 28832688 Free PMC article.
-
Exploring Large Protein Sequence Space through Homology- and Representation-based Hierarchical Clustering.Mol Biol Evol. 2025 Jun 4;42(6):msaf136. doi: 10.1093/molbev/msaf136. Mol Biol Evol. 2025. PMID: 40465845 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 AI043006/AI/NIAID NIH HHS/United States
- HHSN272201200025C/PHS HHS/United States
- HHSN272200700057C/PHS HHS/United States
- R01 AI017828/AI/NIAID NIH HHS/United States
- R01 AI059118/AI/NIAID NIH HHS/United States
- HHSN272200700057C/AI/NIAID NIH HHS/United States
- T32 AI007540/AI/NIAID NIH HHS/United States
- R01AI017828/AI/NIAID NIH HHS/United States
- R01AI043006/AI/NIAID NIH HHS/United States
- T32AI095190/AI/NIAID NIH HHS/United States
- HHSN272201200025C/AI/NIAID NIH HHS/United States
- T32AI007540/AI/NIAID NIH HHS/United States
- R01AI59118/AI/NIAID NIH HHS/United States
- T32 AI095190/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

