Endoglin regulates mural cell adhesion in the circulatory system
- PMID: 26646071
- PMCID: PMC4805714
- DOI: 10.1007/s00018-015-2099-4
Endoglin regulates mural cell adhesion in the circulatory system
Abstract
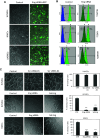
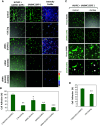
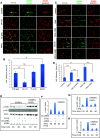
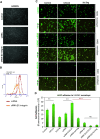
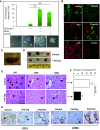
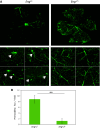
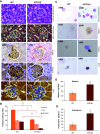
The circulatory system is walled off by different cell types, including vascular mural cells and podocytes. The interaction and interplay between endothelial cells (ECs) and mural cells, such as vascular smooth muscle cells or pericytes, play a pivotal role in vascular biology. Endoglin is an RGD-containing counter-receptor for β1 integrins and is highly expressed by ECs during angiogenesis. We find that the adhesion between vascular ECs and mural cells is enhanced by integrin activators and inhibited upon suppression of membrane endoglin or β1-integrin, as well as by addition of soluble endoglin (SolEng), anti-integrin α5β1 antibody or an RGD peptide. Analysis of different endoglin mutants, allowed the mapping of the endoglin RGD motif as involved in the adhesion process. In Eng (+/-) mice, a model for hereditary hemorrhagic telangectasia type 1, endoglin haploinsufficiency induces a pericyte-dependent increase in vascular permeability. Also, transgenic mice overexpressing SolEng, an animal model for preeclampsia, show podocyturia, suggesting that SolEng is responsible for podocytes detachment from glomerular capillaries. These results suggest a critical role for endoglin in integrin-mediated adhesion of mural cells and provide a better understanding on the mechanisms of vessel maturation in normal physiology as well as in pathologies such as preeclampsia or hereditary hemorrhagic telangiectasia.
Keywords: Blood vessels; Cell adhesion; HHT; Kidney; TGF-β; Tubulogenesis.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

