Screening for transfusion transmissible infections using rapid diagnostic tests in Africa: a potential hazard to blood safety?
- PMID: 26646317
- PMCID: PMC5061037
- DOI: 10.1111/vox.12327
Screening for transfusion transmissible infections using rapid diagnostic tests in Africa: a potential hazard to blood safety?
Abstract
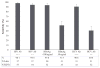
Rapid diagnostic tests (RDTs) are routinely used in African blood centres. We analysed data from two cross-sectional studies representing 95 blood centres in 29 African countries. Standardized panels of sera containing varying concentrations of anti-human immunodeficiency virus (HIV) antibodies (Ab), hepatitis B virus antigen (HBsAg) and antihepatitis C virus (HCV) Ab were screened using routine operational testing procedures at the centres. Sensitivity of detection using RDTs was high for HIV Ab-positive samples, but low for intermediately HBsAg (51·5%) and HCV Ab (40·6%)-positive samples. These findings suggest that current RDT use in Africa could pose a hazard to blood safety.
Keywords: Africa; HIV; blood transfusion; hepatitis B; hepatitis C; rapid diagnostic test.
© 2015 International Society of Blood Transfusion.
Conflict of interest statement
Conflict of interests The authors declare no conflict of interests.
Figures
References
-
- World Health Organization. Global Database on Drug Safety. Geneva: World Health Organisation; 2011. [Last accessed 21 May 2015]. http://www.who.int/bloodsafety/global_database.
-
- Laperche S Francophone African Group for Research in Blood Transfusion. Multinational assessment of blood-borne virus testing and transfusion safety on the African continent. Transfusion. 2013;53:816–826. - PubMed
-
- World Health Organization. Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations. Geneva: World Health Organisation; 2010. - PubMed
-
- Improving blood safety worldwide. Lancet. 2007;370:361. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

