Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials
- PMID: 26646318
- PMCID: PMC4936198
- DOI: 10.1021/acs.chemrev.5b00299
Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials
Abstract
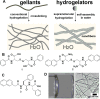
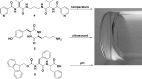
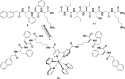
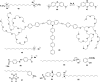
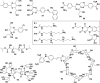
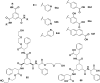
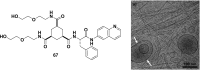
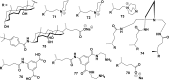
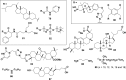
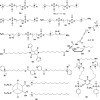
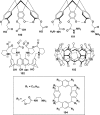
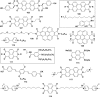
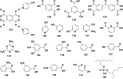
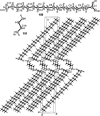
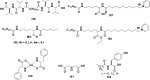
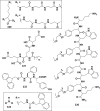
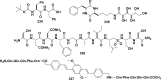
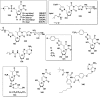
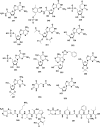
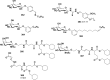
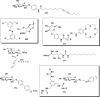
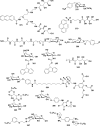
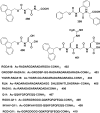
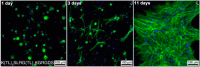
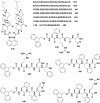
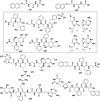
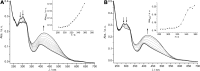
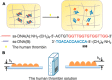
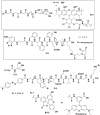
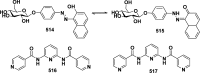
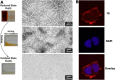
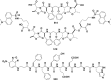
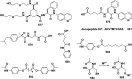
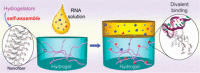
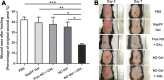
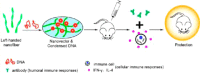
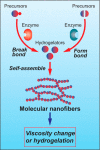
In this review we intend to provide a relatively comprehensive summary of the work of supramolecular hydrogelators after 2004 and to put emphasis particularly on the applications of supramolecular hydrogels/hydrogelators as molecular biomaterials. After a brief introduction of methods for generating supramolecular hydrogels, we discuss supramolecular hydrogelators on the basis of their categories, such as small organic molecules, coordination complexes, peptides, nucleobases, and saccharides. Following molecular design, we focus on various potential applications of supramolecular hydrogels as molecular biomaterials, classified by their applications in cell cultures, tissue engineering, cell behavior, imaging, and unique applications of hydrogelators. Particularly, we discuss the applications of supramolecular hydrogelators after they form supramolecular assemblies but prior to reaching the critical gelation concentration because this subject is less explored but may hold equally great promise for helping address fundamental questions about the mechanisms or the consequences of the self-assembly of molecules, including low molecular weight ones. Finally, we provide a perspective on supramolecular hydrogelators. We hope that this review will serve as an updated introduction and reference for researchers who are interested in exploring supramolecular hydrogelators as molecular biomaterials for addressing the societal needs at various frontiers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
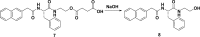

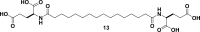

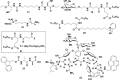
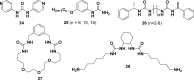
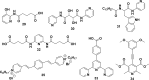




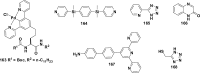
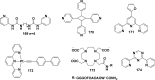
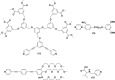
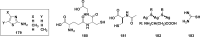

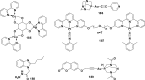
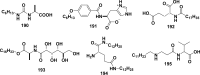
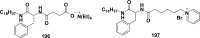

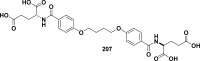


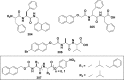

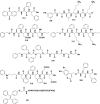
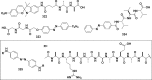
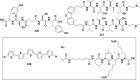






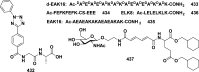






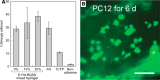
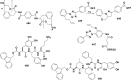
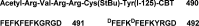
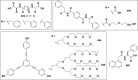
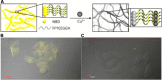

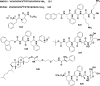
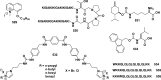
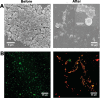
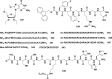


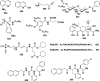
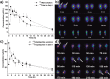
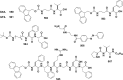




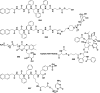




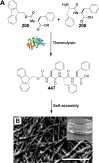

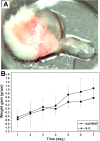



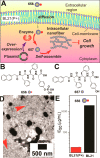
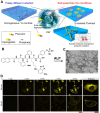

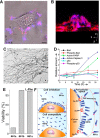

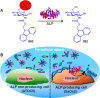

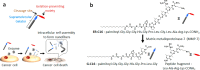
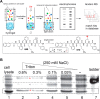
References
-
- Lehn J. M. Perspectives in Supramolecular Chemistry - from Molecular Recognition Towards Molecular Information-Processing and Self-Organization. Angew. Chem., Int. Ed. Engl. 1990, 29, 1304–1319. 10.1002/anie.199013041. - DOI
-
- Luisi P. L. Chemistry Constraints on The Origin of Life. Isr. J. Chem. 2015, 55, 906–918. 10.1002/ijch.201400177. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

