Ulocuplumab (BMS-936564 / MDX1338): a fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway
- PMID: 26646452
- PMCID: PMC4823073
- DOI: 10.18632/oncotarget.6465
Ulocuplumab (BMS-936564 / MDX1338): a fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway
Abstract
The CXCR4 receptor (Chemokine C-X-C motif receptor 4) is highly expressed in different hematological malignancies including chronic lymphocytic leukemia (CLL). The CXCR4 ligand (CXCL12) stimulates CXCR4 promoting cell survival and proliferation, and may contribute to the tropism of leukemia cells towards lymphoid tissues. Therefore, strategies targeting CXCR4 may constitute an effective therapeutic approach for CLL. To address that question, we studied the effect of Ulocuplumab (BMS-936564), a fully human IgG4 anti-CXCR4 antibody, using a stroma--CLL cells co-culture model. We found that Ulocuplumab (BMS-936564) inhibited CXCL12 mediated CXCR4 activation-migration of CLL cells at nanomolar concentrations. This effect was comparable to AMD3100 (Plerixafor--Mozobil), a small molecule CXCR4 inhibitor. However, Ulocuplumab (BMS-936564) but not AMD3100 induced apoptosis in CLL at nanomolar concentrations in the presence or absence of stromal cell support. This pro-apoptotic effect was independent of CLL high-risk prognostic markers, was associated with production of reactive oxygen species and did not require caspase activation. Overall, these findings are evidence that Ulocuplumab (BMS-936564) has biological activity in CLL, highlight the relevance of the CXCR4-CXCL12 pathway as a therapeutic target in CLL, and provide biological rationale for ongoing clinical trials in CLL and other hematological malignancies.
Keywords: BMS-936564; CXCR4; Ulocuplumab; chronic lymphocytic leukemia; reactive oxygen species.
Conflict of interest statement
Kuhne, Sabbatini, Cohen, Shelat, and Cardarelli are employees of Bristol-Myers Squibb. The other authors disclosed no potential conflicts of interests.
Figures


 ,
,  and
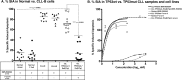
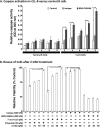
and  represent total binding (TB), non-saturable binding (NSB), and saturable binding (SB), respectively. E. Ramos cells (target) were labeled with bis(acetoxymethyl) 2,2′:6′,2″-terpyridine-6,6″-dicarboxylate (BADTA). Freshly isolated human peripheral blood mononuclear cells PBMCs (effector) were used for allotyping of the Ulocuplumab (BMS-936564). Human PBMCs were cultured with labeled Ramos cells at 1:50 ratio of target/effector cell (T/E) ratios in the presence or absence of rituximab, Ulocuplumab, or isotype (BMS-936564) for 1 h at 37°C. Ramos cells alone served as spontaneous release (SR) and Ramos cells lysed with 1% Triton X-100 served as total release (TR). The lysis was measured by using europium (Eu)-based detection. BMS-936564, Rituximab and their respective isotypes with varied concentrations were tested using Ramos cell line. F. Cell-based CDC assay of Ulocuplumab (BMS-936564) /Rituximab and their respective isotypes as controls: lysis of Ramos cells in the presence of human complement was measured by using Alamar Blue release. G. The stable transfected Jurkat cell line expressing FcgRIIIa and NFAT-RE luc was used as effector E. in an ADCC Reporter Bioassay from Promega. CLL cells (target, T) were plated in ratio of 1:1 with the effector cells. The effector: target cells were incubated in the presence or absence of rituximab or Ulocuplumab (BMS-936564), or obinutuzumab between 0.001-30 ug/ml of concentrations for 6 hrs at 37°C. Rituximab and obinutuzumab were used as positive controls. The reaction was developed by incubating the cells with Bio-GloTM reagent for 30 minutes at room temperature in dark. The plates were read on luminometer and following the background subtraction, relative-light units (RLU) were calculated for different antibodies/isotypes using GraphPad Prism software. H. Complement dependent cytotoxicity (CDC) for 10 μg/ml of either Ulocuplumab (BMS-936564), rituximab, obinutuzumab or isotype was tested in CLL cells after incubation with either 5% fresh human or heat inactivated serum (to denature complement) was measured by using CD19/CD5/Annexin V staining followed by flow cytomerty analysis. Rituximab and obinutuzumab were used as positive controls. The data are the mean and SD of triplicate cultures. The statistical data was analyzed using Bonferroni correction test in GraphPad Prism software.
represent total binding (TB), non-saturable binding (NSB), and saturable binding (SB), respectively. E. Ramos cells (target) were labeled with bis(acetoxymethyl) 2,2′:6′,2″-terpyridine-6,6″-dicarboxylate (BADTA). Freshly isolated human peripheral blood mononuclear cells PBMCs (effector) were used for allotyping of the Ulocuplumab (BMS-936564). Human PBMCs were cultured with labeled Ramos cells at 1:50 ratio of target/effector cell (T/E) ratios in the presence or absence of rituximab, Ulocuplumab, or isotype (BMS-936564) for 1 h at 37°C. Ramos cells alone served as spontaneous release (SR) and Ramos cells lysed with 1% Triton X-100 served as total release (TR). The lysis was measured by using europium (Eu)-based detection. BMS-936564, Rituximab and their respective isotypes with varied concentrations were tested using Ramos cell line. F. Cell-based CDC assay of Ulocuplumab (BMS-936564) /Rituximab and their respective isotypes as controls: lysis of Ramos cells in the presence of human complement was measured by using Alamar Blue release. G. The stable transfected Jurkat cell line expressing FcgRIIIa and NFAT-RE luc was used as effector E. in an ADCC Reporter Bioassay from Promega. CLL cells (target, T) were plated in ratio of 1:1 with the effector cells. The effector: target cells were incubated in the presence or absence of rituximab or Ulocuplumab (BMS-936564), or obinutuzumab between 0.001-30 ug/ml of concentrations for 6 hrs at 37°C. Rituximab and obinutuzumab were used as positive controls. The reaction was developed by incubating the cells with Bio-GloTM reagent for 30 minutes at room temperature in dark. The plates were read on luminometer and following the background subtraction, relative-light units (RLU) were calculated for different antibodies/isotypes using GraphPad Prism software. H. Complement dependent cytotoxicity (CDC) for 10 μg/ml of either Ulocuplumab (BMS-936564), rituximab, obinutuzumab or isotype was tested in CLL cells after incubation with either 5% fresh human or heat inactivated serum (to denature complement) was measured by using CD19/CD5/Annexin V staining followed by flow cytomerty analysis. Rituximab and obinutuzumab were used as positive controls. The data are the mean and SD of triplicate cultures. The statistical data was analyzed using Bonferroni correction test in GraphPad Prism software.


References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. - PubMed
-
- Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human Protein Reference Database--2009 update. Nucleic acids research. 2009;37:D767–772. - PMC - PubMed
-
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5215–5220. - PMC - PubMed
-
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunological reviews. 2000;177:175–184. - PubMed
-
- Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical cancer research. 2010;16:2927–2931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

