Placental growth factor deficiency is associated with impaired cerebral vascular development in mice
- PMID: 26646502
- PMCID: PMC4733225
- DOI: 10.1093/molehr/gav069
Placental growth factor deficiency is associated with impaired cerebral vascular development in mice
Abstract
Study hypothesis: Placental growth factor (PGF) is expressed in the developing mouse brain and contributes to vascularization and vessel patterning.
Study finding: PGF is dynamically expressed in fetal mouse brain, particularly forebrain, and is essential for normal cerebrovascular development.
What is known already: PGF rises in maternal plasma over normal human and mouse pregnancy but is low in many women with the acute onset hypertensive syndrome, pre-eclampsia (PE). Little is known about the expression of PGF in the fetus during PE. Pgf (-/-) mice appear normal but recently cerebral vascular defects were documented in adult Pgf (-/-) mice.
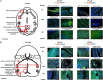
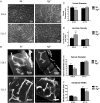
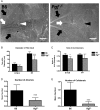
Study design, samples/materials, methods: Here, temporal-spatial expression of PGF is mapped in normal fetal mouse brains and cerebral vasculature development is compared between normal and congenic Pgf (-/-) fetuses to assess the actions of PGF during cerebrovascular development. Pgf/PGF, Vegfa/VEGF, Vegf receptor (Vegfr)1 and Vegfr2 expression were examined in the brains of embryonic day (E)12.5, 14.5, 16.5 and 18.5 C57BL/6 (B6) mice using quantitative PCR and immunohistochemistry. The cerebral vasculature was compared between Pgf (-/-) and B6 embryonic and adult brains using whole mount techniques. Vulnerability to cerebral ischemia was investigated using a left common carotid ligation assay.
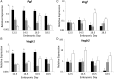
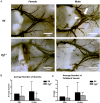
Main results and the role of chance: Pgf/PGF and Vegfr1 are highly expressed in E12.5-14.5 forebrain relative to VEGF and Vegfr2. Vegfa/VEGF is relatively more abundant in hindbrain (HB). PGF and VEGF expression were similar in midbrain. Delayed HB vascularization was seen at E10.5 and 11.5 in Pgf (-/-) brains. At E14.5, Pgf (-/-) circle of Willis showed unilateral hypoplasia and fewer collateral vessels, defects that persisted post-natally. Functionally, adult Pgf (-/-) mice experienced cerebral ischemia after left common carotid arterial occlusion while B6 mice did not.
Limitations, reasons for caution: Since Pgf (-/-) mice were used, consequences of complete absence of maternal and fetal PGF were defined. Therefore, the effects of maternal versus fetal PGF deficiency on cerebrovascular development cannot be separated. However, as PGF was strongly expressed in the developing brain at all timepoints, we suggest that local PGF has a more important role than distant maternal or placental sources. Full PGF loss is not expected in PE pregnancies, predicting that the effects of PGF deficiency identified in this model will be more severe than any effects in PE-offspring.
Wider implications of the findings: These studies provoke the question of whether PGF expression is decreased and cerebral vascular maldevelopment occurs in fetuses who experience a preeclamptic gestation. These individuals have already been reported to have elevated risk for stroke and cognitive impairments.
Large scale data: N/A.
Study funding and competing interests: This work was supported by awards from the Natural Sciences and Engineering Research Council, the Canada Research Chairs Program and the Canadian Foundation for Innovation to B.A.C. and by training awards from the Universidade Federal de Pernambuco and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brazil to R.L.L.; Queen's University to V.R.K. and the Canadian Institutes of Health Research to M.T.R. The work of P.C. is supported by the Belgian Science Policy BELSPO-IUAP7/03, Structural funding by the Flemish Government-Methusalem funding, and the Flemish Science Fund-FWO grants. There were no competing interests.
Keywords: PGF; cerebral vessels; circle of Willis; fetal development; pre-eclampsia; stroke.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat.Mol Hum Reprod. 2017 Jul 1;23(7):509-519. doi: 10.1093/molehr/gax024. Mol Hum Reprod. 2017. PMID: 28402512
-
Placental expression of VEGF family mRNA in adverse pregnancy outcomes.Placenta. 2012 Jun;33(6):467-72. doi: 10.1016/j.placenta.2012.02.013. Epub 2012 Mar 3. Placenta. 2012. PMID: 22386962
-
Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences.J Clin Endocrinol Metab. 2003 Nov;88(11):5555-63. doi: 10.1210/jc.2003-030528. J Clin Endocrinol Metab. 2003. PMID: 14602804
-
Preeclampsia may influence offspring neuroanatomy and cognitive function: a role for placental growth factor†.Biol Reprod. 2019 Aug 1;101(2):271-283. doi: 10.1093/biolre/ioz095. Biol Reprod. 2019. PMID: 31175349 Review.
-
Expression and function of placenta growth factor: implications for abnormal placentation.J Soc Gynecol Investig. 2003 May;10(4):178-88. doi: 10.1016/s1071-5576(03)00048-0. J Soc Gynecol Investig. 2003. PMID: 12759145 Review.
Cited by
-
Diffusion Tensor Imaging of White Matter in Children Born from Preeclamptic Gestations.AJNR Am J Neuroradiol. 2017 Apr;38(4):801-806. doi: 10.3174/ajnr.A5064. Epub 2017 Jan 26. AJNR Am J Neuroradiol. 2017. PMID: 28126749 Free PMC article.
-
Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring.JAMA Psychiatry. 2020 Aug 1;77(8):823-829. doi: 10.1001/jamapsychiatry.2020.0306. JAMA Psychiatry. 2020. PMID: 32236510 Free PMC article.
-
Anatomical variations in the circle of Willis on magnetic resonance angiography in a south Trinidad population.BJR Open. 2023 Dec 12;6(1):tzad002. doi: 10.1093/bjro/tzad002. eCollection 2024 Jan. BJR Open. 2023. PMID: 38352180 Free PMC article.
-
Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice.Physiol Genomics. 2018 Oct 1;50(10):862-875. doi: 10.1152/physiolgenomics.00076.2018. Epub 2018 Aug 17. Physiol Genomics. 2018. PMID: 30118404 Free PMC article.
-
RNA binding protein, tristetraprolin in a murine model of recurrent pregnancy loss.Oncotarget. 2016 Nov 8;7(45):72486-72502. doi: 10.18632/oncotarget.12539. Oncotarget. 2016. PMID: 27732963 Free PMC article.
References
-
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F et al. . Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 2003;7:936–943. - PubMed
-
- Baston-Buest DM, Porn AC, Schanz A, Kruessel JC, Janni W, Hess AP. Expression of the vascular endothelial growth factor receptor neuropilin-1 at the human embryo-maternal interface. Eur J Obstet Gynecol Reprod Biol 2011;2:151–156. - PubMed
-
- Beck H, Acker T, Püschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol 2002;4:339–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

