Interleukin-13 Inhibits Lipopolysaccharide-Induced BPIFA1 Expression in Nasal Epithelial Cells
- PMID: 26646664
- PMCID: PMC4672888
- DOI: 10.1371/journal.pone.0143484
Interleukin-13 Inhibits Lipopolysaccharide-Induced BPIFA1 Expression in Nasal Epithelial Cells
Abstract
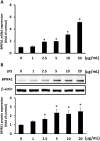
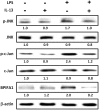
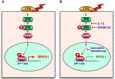
Short palate, lung, and nasal epithelium clone 1 (SPLUNC1) protein is expressed in human nasopharyngeal and respiratory epithelium and has demonstrated antimicrobial activity. SPLUNC1 is now referred to as bactericidal/permeability-increasing fold containing family A, member 1 (BPIFA1). Reduced BPIFA1 expression is associated with bacterial colonization in patients with chronic rhinosinusitis with nasal polyps (CRSwNP). Interleukin 13 (IL-13), predominately secreted by T helper 2 (TH2) cells, has been found to contribute to airway allergies and suppress BPIFA1 expression in nasal epithelial cells. However, the molecular mechanism of IL-13 perturbation of bacterial infection and BPIFA1 expression in host airways remains unclear. In this study, we found that lipopolysaccharide (LPS)-induced BPIFA1 expression in nasal epithelial cells was mediated through the JNK/c-Jun signaling pathway and AP-1 activation. We further demonstrated that IL-13 downregulated the LPS-induced activation of phosphorylated JNK and c-Jun, followed by attenuation of BPIFA1 expression. Moreover, the immunohistochemical analysis showed that IL-13 prominently suppressed BPIFA1 expression in eosinophilic CRSwNP patients with bacterial infection. Taken together, these results suggest that IL-13 plays a critical role in attenuation of bacteria-induced BPIFA1 expression that may result in eosinophilic CRSwNP.
Conflict of interest statement
Figures





References
-
- Di YP, Harper R, Zhao Y, Pahlavan N, Finkbeiner W, Wu R. Molecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tracts. J Biol Chem 2003;278: 1165–1173. - PubMed
-
- Bingle CD, Gorr SU. Host defense in oral and airway epithelia: chromosome 20 contributes a new protein family. Int J Biochem Cell Biol 2004;36: 2144–2152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

