Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea
- PMID: 26647161
- PMCID: PMC6417502
- DOI: 10.1016/j.survophthal.2015.11.006
Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea
Abstract
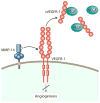
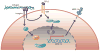
The cornea is transparent and avascular, and retention of these characteristics is critical to maintaining vision clarity. Under normal conditions, wound healing in response to corneal injury occurs without the formation of new blood vessels; however, neovascularization may be induced during corneal wound healing when the balance between proangiogenic and antiangiogenic mediators is disrupted to favor angiogenesis. Matrix metalloproteinases (MMPs), which are key factors in extracellular matrix remodeling and angiogenesis, contribute to the maintenance of this balance, and in pathologic instances, can contribute to its disruption. Here, we elaborate on the facilitative role of MMPs, specifically MMP-14, in corneal neovascularization. MMP-14 is a transmembrane MMP that is critically involved in extracellular matrix proteolysis, exosome transport, and cellular migration and invasion, processes that are critical for angiogenesis. To aid in developing efficacious therapies that promote healing without neovascularization, it is important to understand and further investigate the complex pathways related to MMP-14 signaling, which can also involve vascular endothelial growth factor, basic fibroblast growth factor, Wnt/β-catenin, transforming growth factor, platelet-derived growth factor, hepatocyte growth factor or chemokines, epidermal growth factor, prostaglandin E2, thrombin, integrins, Notch, Toll-like receptors, PI3k/Akt, Src, RhoA/RhoA kinase, and extracellular signal-related kinase. The involvement and potential contribution of these signaling molecules or proteins in neovascularization are the focus of the present review.
Keywords: MMP-14; VEGF-A; bFGF; corneal neovascularization; signal transduction.
Published by Elsevier Inc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Alfranca A, Lopez-Oliva JM, Genis L, et al. PGE2 induces angiogenesis via MT1-MMP-mediated activation of the TGFbeta/Alk5 signaling pathway. Blood. 2008;112:1120–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

