Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease?
- PMID: 26647358
- PMCID: PMC4847635
- DOI: 10.1194/jlr.R060582
Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease?
Abstract
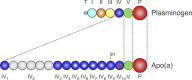
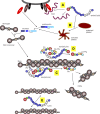
Elevated plasma concentrations of lipoprotein (a) [Lp(a)] have been determined to be a causal risk factor for coronary heart disease, and may similarly play a role in other atherothrombotic disorders. Lp(a) consists of a lipoprotein moiety indistinguishable from LDL, as well as the plasminogen-related glycoprotein, apo(a). Therefore, the pathogenic role for Lp(a) has traditionally been considered to reflect a dual function of its similarity to LDL, causing atherosclerosis, and its similarity to plasminogen, causing thrombosis through inhibition of fibrinolysis. This postulate remains highly speculative, however, because it has been difficult to separate the prothrombotic/antifibrinolytic functions of Lp(a) from its proatherosclerotic functions. This review surveys the current landscape surrounding these issues: the biochemical basis for procoagulant and antifibrinolytic effects of Lp(a) is summarized and the evidence addressing the role of Lp(a) in both arterial and venous thrombosis is discussed. While elevated Lp(a) appears to be primarily predisposing to thrombotic events in the arterial tree, the fact that most of these are precipitated by underlying atherosclerosis continues to confound our understanding of the true pathogenic roles of Lp(a) and, therefore, the most appropriate therapeutic target through which to mitigate the harmful effects of this lipoprotein.
Keywords: apolipoprotein (a); apolipoproteins; atherosclerosis; coagulation; fibrinolysis; lipoproteins; lipoproteins/kinetics; low density lipoprotein; thrombosis.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Berg K., Dahlén G., and Frick M. H.. 1974. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin. Genet. 6: 230–235. - PubMed
-
- Dahlén G., Berg K., Gillnäs T., and Ericson C.. 1975. Lp(a) lipoprotein/pre-beta1-lipoprotein in Swedish middle-aged males and in patients with coronary heart disease. Clin. Genet. 7: 334–341. - PubMed
-
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., and Lawn R. M.. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. - PubMed
-
- Miles L. A., Fless G. M., Levin E. G., Scanu A. M., and Plow E. F.. 1989. A potential basis for the thrombotic risks associated with lipoprotein(a). Nature. 339: 301–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

