Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials
- PMID: 26647432
- PMCID: PMC4697914
- DOI: 10.1183/13993003.00026-2015
Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials
Abstract
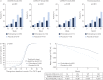
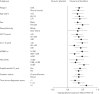
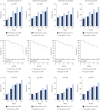
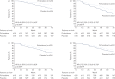
Pirfenidone is an antifibrotic agent that has been evaluated in three multinational phase 3 trials in patients with idiopathic pulmonary fibrosis (IPF). We analysed pooled data from the multinational trials to obtain the most precise estimates of the magnitude of treatment effect on measures of disease progression.All patients randomised to pirfenidone 2403 mg·day(-1) or placebo in the CAPACITY or ASCEND studies were included in the analysis. Pooled analyses of outcomes at 1 year were based on the pre-specified end-points and analytic methods described in the ASCEND study protocol.A total of 1247 patients were included in the analysis. At 1 year, pirfenidone reduced the proportion of patients with a ≥10% decline in per cent predicted forced vital capacity or death by 43.8% (95% CI 29.3-55.4%) and increased the proportion of patients with no decline by 59.3% (95% CI 29.0-96.8%). A treatment benefit was also observed for progression-free survival, 6-min walk distance and dyspnoea. Gastrointestinal and skin-related adverse events were more common in the pirfenidone group, but rarely led to discontinuation.Analysis of data from three phase 3 trials demonstrated that treatment with pirfenidone for 1 year resulted in clinically meaningful reductions in disease progression in patients with IPF.
Copyright ©ERS 2016.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Figures


Comment in
-
Therapy for idiopathic pulmonary fibrosis: lessons from pooled data analyses.Eur Respir J. 2016 Jan;47(1):27-30. doi: 10.1183/13993003.01669-2015. Eur Respir J. 2016. PMID: 26721960 No abstract available.
-
Pirfenidone for Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2016 Aug 1;194(3):374-6. doi: 10.1164/rccm.201602-0258RR. Am J Respir Crit Care Med. 2016. PMID: 27244589 No abstract available.
References
-
- Noble PW, Albera C, Bradford WZ, et al. . Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. - PubMed
-
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. - PubMed
-
- Taniguchi H, Ebina M, Kondoh Y, et al. . Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 821–829. - PubMed
-
- Azuma A, Nukiwa T, Tsuboi E, et al. . Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. - PubMed
-
- Spagnolo P, Del Giovane C, Luppi F, et al. . Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2010; 9: CD003134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
