Subverting Toll-Like Receptor Signaling by Bacterial Pathogens
- PMID: 26648936
- PMCID: PMC4664646
- DOI: 10.3389/fimmu.2015.00607
Subverting Toll-Like Receptor Signaling by Bacterial Pathogens
Abstract
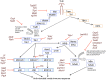
Pathogenic bacteria are detected by pattern-recognition receptors (PRRs) expressed on innate immune cells, which activate intracellular signal transduction pathways to elicit an immune response. Toll-like receptors are, perhaps, the most studied of the PRRs and can activate the mitogen-activated protein kinase (MAPK) and Nuclear Factor-κB (NF-κB) pathways. These pathways are critical for mounting an effective immune response. In order to evade detection and promote virulence, many pathogens subvert the host immune response by targeting components of these signal transduction pathways. This mini-review highlights the diverse mechanisms that bacterial pathogens have evolved to manipulate the innate immune response, with a particular focus on those that target MAPK and NF-κB signaling pathways. Understanding the elaborate strategies that pathogens employ to subvert the immune response not only highlights the importance of these proteins in mounting effective immune responses, but may also identify novel approaches for treatment or prevention of infection.
Keywords: MAPK; NF-κB; TLR; bacterial effector; bacterial pathogen; signaling; virulence.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

