IFN-γ-Producing T-Helper 17.1 Cells Are Increased in Sarcoidosis and Are More Prevalent than T-Helper Type 1 Cells
- PMID: 26649486
- PMCID: PMC4910899
- DOI: 10.1164/rccm.201507-1499OC
IFN-γ-Producing T-Helper 17.1 Cells Are Increased in Sarcoidosis and Are More Prevalent than T-Helper Type 1 Cells
Abstract
Rationale: Pulmonary sarcoidosis is classically defined by T-helper (Th) cell type 1 inflammation (e.g., IFN-γ production by CD4(+) effector T cells). Recently, IL-17A-secreting cells have been found in lung lavage, invoking Th17 immunity in sarcoidosis. Studies also identified IL-17A-secreting cells that expressed IFN-γ, but their abundance as a percentage of total CD4(+) cells was either low or undetermined.
Objectives: Based on evidence that Th17 cells can be polarized to Th17.1 cells to produce only IFN-γ, our goal was to determine whether Th17.1 cells are a prominent source of IFN-γ in sarcoidosis.
Methods: We developed a single-cell approach to define and isolate major Th-cell subsets using combinations of chemokine receptors and fluorescence-activated cell sorting. We subsequently confirmed the accuracy of subset enrichment by measuring cytokine production.
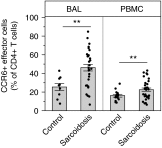
Measurements and main results: Discrimination between Th17 and Th17.1 cells revealed very high percentages of Th17.1 cells in lung lavage in sarcoidosis compared with controls in two separate cohorts. No differences in Th17 or Th1 lavage cells were found compared with controls. Lung lavage Th17.1-cell percentages were also higher than Th1-cell percentages, and approximately 60% of Th17.1-enriched cells produced only IFN-γ.
Conclusions: Combined use of surface markers and functional assays to study CD4(+) T cells in sarcoidosis revealed a marked expansion of Th17.1 cells that only produce IFN-γ. These results suggest that Th17.1 cells could be misclassified as Th1 cells and may be the predominant producer of IFN-γ in pulmonary sarcoidosis, challenging the Th1 paradigm of pathogenesis.
Keywords: chemokine receptor; inflammation; lymphocyte.
Figures




Comment in
-
Sarcoidosis and T-Helper Cells. Th1, Th17, or Th17.1?Am J Respir Crit Care Med. 2016 Jun 1;193(11):1198-200. doi: 10.1164/rccm.201512-2419ED. Am J Respir Crit Care Med. 2016. PMID: 27248588 Free PMC article. No abstract available.
References
-
- Chen ES, Moller DR. Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. - PubMed
-
- Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305:429–434. - PubMed
-
- Agostini C, Meneghin A, Semenzato G. T-lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8:435–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

