Importance of Hydrophilic Hydration and Intramolecular Interactions in the Thermodynamics of Helix-Coil Transition and Helix-Helix Assembly in a Deca-Alanine Peptide
- PMID: 26649757
- PMCID: PMC5321054
- DOI: 10.1021/acs.jpcb.5b09881
Importance of Hydrophilic Hydration and Intramolecular Interactions in the Thermodynamics of Helix-Coil Transition and Helix-Helix Assembly in a Deca-Alanine Peptide
Abstract
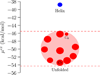
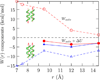
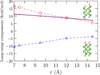
For a model deca-alanine peptide the cavity (ideal hydrophobic) contribution to hydration favors the helix state over extended states and the paired helix bundle in the assembly of two helices. The energetic contributions of attractive protein-solvent interactions are separated into quasi-chemical components consisting of a short-range part arising from interactions with solvent in the first hydration shell and the remaining long-range part that is well described by a Gaussian. In the helix-coil transition, short-range attractive protein-solvent interactions outweigh hydrophobic hydration and favor the extended coil states. Analysis of enthalpic effects shows that it is the favorable hydration of the peptide backbone that favors the unfolded state. Protein intramolecular interactions favor the helix state and are decisive in favoring folding. In the pairing of two helices, the cavity contribution outweighs the short-range attractive protein-water interactions. However, long-range, protein-solvent attractive interactions can either enhance or reverse this trend depending on the mutual orientation of the helices. In helix-helix assembly, change in enthalpy arising from change in attractive protein-solvent interactions favors disassembly. In helix pairing as well, favorable protein intramolecular interactions are found to be as important as hydration effects. Overall, hydrophilic protein-solvent interactions and protein intramolecular interactions are found to play a significant role in the thermodynamics of folding and assembly in the system studied.
Figures

Similar articles
-
Solvophobic and solvophilic contributions in the water-to-aqueous guanidinium chloride transfer free energy of model peptides.J Chem Phys. 2018 Jun 14;148(22):222822. doi: 10.1063/1.5022465. J Chem Phys. 2018. PMID: 29907034
-
Free energy determinants of secondary structure formation: I. alpha-Helices.J Mol Biol. 1995 Sep 22;252(3):351-65. doi: 10.1006/jmbi.1995.0502. J Mol Biol. 1995. PMID: 7563056
-
Alpha-helix stabilization by alanine relative to glycine: roles of polar and apolar solvent exposures and of backbone entropy.Proteins. 2006 Aug 15;64(3):769-78. doi: 10.1002/prot.21041. Proteins. 2006. PMID: 16755589
-
Helices and Sheets in vacuo.Phys Chem Chem Phys. 2007 Apr 14;9(14):1659-71. doi: 10.1039/b612615d. Epub 2007 Jan 19. Phys Chem Chem Phys. 2007. PMID: 17396176 Review.
-
Dynamics of hydration water in proteins.Gen Physiol Biophys. 2009 Jun;28(2):168-73. doi: 10.4149/gpb_2009_02_168. Gen Physiol Biophys. 2009. PMID: 19592713 Review.
Cited by
-
Predicting unfolding thermodynamics and stable intermediates for alanine-rich helical peptides with the aid of coarse-grained molecular simulation.Biophys Chem. 2016 Oct;217:8-19. doi: 10.1016/j.bpc.2016.07.002. Epub 2016 Jul 22. Biophys Chem. 2016. PMID: 27486699 Free PMC article.
-
Analysis of nonsynonymous SNPs in candidate genes that influence bovine temperament and evaluation of their effect in Brahman cattle.Mol Biol Rep. 2024 Feb 7;51(1):285. doi: 10.1007/s11033-024-09264-4. Mol Biol Rep. 2024. PMID: 38324050 Free PMC article.
-
Statistical Mechanical Design Principles for Coarse-Grained Interactions across Different Conformational Free Energy Surfaces.J Phys Chem Lett. 2023 Feb 16;14(6):1354-1362. doi: 10.1021/acs.jpclett.2c03844. Epub 2023 Feb 2. J Phys Chem Lett. 2023. PMID: 36728761 Free PMC article.
-
A Structural Analysis of Proteinaceous Nanotube Cavities and Their Applications in Nanotechnology.Nanomaterials (Basel). 2022 Nov 20;12(22):4080. doi: 10.3390/nano12224080. Nanomaterials (Basel). 2022. PMID: 36432365 Free PMC article. Review.
-
Intramolecular Interactions Overcome Hydration to Drive the Collapse Transition of Gly15.J Phys Chem B. 2017 Aug 31;121(34):8078-8084. doi: 10.1021/acs.jpcb.7b05469. Epub 2017 Aug 21. J Phys Chem B. 2017. PMID: 28774177 Free PMC article.
References
-
- Weber V, Merchant S, Asthagiri D. Regularizing Binding Energy Distributions And Thermodynamics Of Hydration: Theory And Application To Water Modeled With Classical And Ab Initio Simulations. J. Chem. Phys. 2011;135:181101. - PubMed
-
- Weber V, Asthagiri D. Regularizing Binding Energy Distributions And The Hydration Free Energy of Protein Cytochrome C From All-Atom Simulations. J. Chem. Theory Comput. 2012;8:3409–3415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

