Biophysical Characterization of G-Quadruplex Recognition in the PITX1 mRNA by the Specificity Domain of the Helicase RHAU
- PMID: 26649896
- PMCID: PMC4674103
- DOI: 10.1371/journal.pone.0144510
Biophysical Characterization of G-Quadruplex Recognition in the PITX1 mRNA by the Specificity Domain of the Helicase RHAU
Abstract
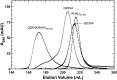
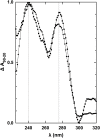
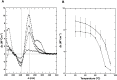
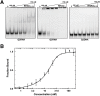
Nucleic acids rich in guanine are able to fold into unique structures known as G-quadruplexes. G-quadruplexes consist of four tracts of guanylates arranged in parallel or antiparallel strands that are aligned in stacked G-quartet planes. The structure is further stabilized by Hoogsteen hydrogen bonds and monovalent cations centered between the planes. RHAU (RNA helicase associated with AU-rich element) is a member of the ATP-dependent DExH/D family of RNA helicases and can bind and resolve G-quadruplexes. RHAU contains a core helicase domain with an N-terminal extension that enables recognition and full binding affinity to RNA and DNA G-quadruplexes. PITX1, a member of the bicoid class of homeobox proteins, is a transcriptional activator active during development of vertebrates, chiefly in the anterior pituitary gland and several other organs. We have previously demonstrated that RHAU regulates PITX1 levels through interaction with G-quadruplexes at the 3'-end of the PITX1 mRNA. To understand the structural basis of G-quadruplex recognition by RHAU, we characterize a purified minimal PITX1 G-quadruplex using a variety of biophysical techniques including electrophoretic mobility shift assays, UV-VIS spectroscopy, circular dichroism, dynamic light scattering, small angle X-ray scattering and nuclear magnetic resonance spectroscopy. Our biophysical analysis provides evidence that the RNA G-quadruplex, but not its DNA counterpart, can adopt a parallel orientation, and that only the RNA can interact with N-terminal domain of RHAU via the tetrad face of the G-quadruplex. This work extends our insight into how the N-terminal region of RHAU recognizes parallel G-quadruplexes.
Conflict of interest statement
Figures




References
-
- Hoogsteen K. Crystal and Molecular Structure of a Hydrogen-Bonded Complex between 1-Methylthymine and 9-Methyladenine. Acta Crystallogr. 1963;16(9):907 10.1107/S0365110x63002437 WOS:A19631326A00007. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

