GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function
- PMID: 26650351
- PMCID: PMC4758954
- DOI: 10.7554/eLife.08881
GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function
Abstract
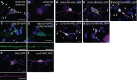
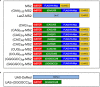
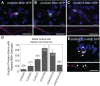
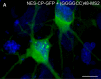
Microsatellite expansions are the leading cause of numerous neurodegenerative disorders. Here we demonstrate that GGGGCC and CAG microsatellite repeat RNAs associated with C9orf72 in amyotrophic lateral sclerosis/frontotemporal dementia and with polyglutamine diseases, respectively, localize to neuritic granules that undergo active transport into distal neuritic segments. In cultured mammalian spinal cord neurons, the presence of neuritic GGGGCC repeat RNA correlates with neuronal branching defects, and the repeat RNA localizes to granules that label with fragile X mental retardation protein (FMRP), a transport granule component. Using a Drosophila GGGGCC expansion disease model, we characterize dendritic branching defects that are modulated by FMRP and Orb2. The human orthologs of these modifiers are misregulated in induced pluripotent stem cell-differentiated neurons (iPSNs) from GGGGCC expansion carriers. These data suggest that expanded repeat RNAs interact with the messenger RNA transport and translation machinery, causing transport granule dysfunction. This could be a novel mechanism contributing to the neuronal defects associated with C9orf72 and other microsatellite expansion diseases.
Keywords: ALS; D. melanogaster; FMRP; FTD; GGGGCC; c9orf72; cell biology; spinal cord neurons.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





References
-
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SSW, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, Clare AJ, Badders NM, Bilican B, Chaum E, Chandran S, Shaw CE, Eggan KC, Maniatis T, Taylor JP. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. - DOI - PMC - PubMed
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, DeGroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao F-B. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathologica. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. - DOI - PMC - PubMed
-
- Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin W-L, DeJesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS073660/NS/NINDS NIH HHS/United States
- R01NS052325/NS/NINDS NIH HHS/United States
- R21NS077909/NS/NINDS NIH HHS/United States
- R21 NS077909/NS/NINDS NIH HHS/United States
- R00 MH090237/MH/NIMH NIH HHS/United States
- R21 AG042179/AG/NIA NIH HHS/United States
- R01NS079725/NS/NINDS NIH HHS/United States
- F32 NS067902/NS/NINDS NIH HHS/United States
- R01 NS073660/NS/NINDS NIH HHS/United States
- R01 NS079725/NS/NINDS NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- F32NS067902/NS/NINDS NIH HHS/United States
- R01 NS052325/NS/NINDS NIH HHS/United States
- T32AG000255/AG/NIA NIH HHS/United States
- R00MH090237/MH/NIMH NIH HHS/United States
- R21AG042179/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

