Precise assembly of complex beta sheet topologies from de novo designed building blocks
- PMID: 26650357
- PMCID: PMC4737653
- DOI: 10.7554/eLife.11012
Precise assembly of complex beta sheet topologies from de novo designed building blocks
Abstract
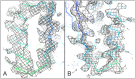
Design of complex alpha-beta protein topologies poses a challenge because of the large number of alternative packing arrangements. A similar challenge presumably limited the emergence of large and complex protein topologies in evolution. Here, we demonstrate that protein topologies with six and seven-stranded beta sheets can be designed by insertion of one de novo designed beta sheet containing protein into another such that the two beta sheets are merged to form a single extended sheet, followed by amino acid sequence optimization at the newly formed strand-strand, strand-helix, and helix-helix interfaces. Crystal structures of two such designs closely match the computational design models. Searches for similar structures in the SCOP protein domain database yield only weak matches with different beta sheet connectivities. A similar beta sheet fusion mechanism may have contributed to the emergence of complex beta sheets during natural protein evolution.
Keywords: E. coli; beta sheets; biophysics; computational biology; protein design; protein folding; structural biology; systems biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







References
-
- Ay J, Gotz F, Borriss R, Heinemann U. Structure and function of the bacillus hybrid enzyme GluXyn-1: native-like jellyroll fold preserved after insertion of autonomous globular domain. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6613–6618. doi: 10.1073/pnas.95.12.6613. - DOI - PMC - PubMed
-
- Ben-Tal N, Kolodny R. Representation of the protein universe using classifications, maps, and networks. Israel Journal of Chemistry. 2014;54:1286–1292. doi: 10.1002/ijch.201400001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

