Bone Marrow-derived Cells Contribute to the Pathogenesis of Pulmonary Arterial Hypertension
- PMID: 26651104
- PMCID: PMC4849178
- DOI: 10.1164/rccm.201502-0407OC
Bone Marrow-derived Cells Contribute to the Pathogenesis of Pulmonary Arterial Hypertension
Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a progressive lung disease of the pulmonary microvasculature. Studies suggest that bone marrow (BM)-derived circulating cells may play an important role in its pathogenesis.
Objectives: We used a genetic model of PAH, the Bmpr2 mutant mouse, to study the role of BM-derived circulating cells in its pathogenesis.
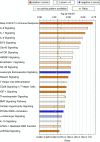
Methods: Recipient mice, either Bmpr2(R899X) mutant or controls, were lethally irradiated and transplanted with either control or Bmpr2(R899X) BM cells. Donor cells were traced in female recipient mice by Y chromosome painting. Molecular and function insights were provided by expression and cytokine arrays combined with flow cytometry, colony-forming assays, and competitive transplant assays.
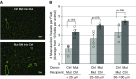
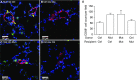
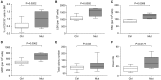
Measurements and main results: We found that mutant BM cells caused PAH with remodeling and inflammation when transplanted into control mice, whereas control BM cells had a protective effect against the development of disease, when transplanted into mutant mice. Donor BM-derived cells were present in the lungs of recipient mice. Functional and molecular analysis identified mutant BM cell dysfunction suggestive of a PAH phenotype soon after activation of the transgene and long before the development of lung pathology.
Conclusions: Our data show that BM cells played a key role in PAH pathogenesis and that the transplanted BM cells were able to drive the lung phenotype in a myeloablative transplant model. Furthermore, the specific cell types involved were derived from hematopoietic stem cells and exhibit dysfunction long before the development of lung pathology.
Keywords: Bmpr2; bone marrow cells; hematopoietic stem cells; pulmonary arterial hypertension; transplantation.
Figures





Comment in
-
The Role of Bone Marrow-derived Cells in Pulmonary Arterial Hypertension. What Lies Beneath?Am J Respir Crit Care Med. 2016 Apr 15;193(8):822-4. doi: 10.1164/rccm.201511-2293ED. Am J Respir Crit Care Med. 2016. PMID: 27082534 Free PMC article. No abstract available.
-
Myeloid Targets for Pulmonary Arterial Hypertension: Time for Another Look.Am J Respir Crit Care Med. 2016 Aug 1;194(3):384. doi: 10.1164/rccm.201603-0465LE. Am J Respir Crit Care Med. 2016. PMID: 27479064 Free PMC article. No abstract available.
-
Reply: Expanded Role for Bone Marrow-derived Hematopoietic Stem and Progenitor Cells in Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2016 Aug 1;194(3):384-5. doi: 10.1164/rccm.201603-0611LE. Am J Respir Crit Care Med. 2016. PMID: 27479065 Free PMC article. No abstract available.
Similar articles
-
Endothelial to haematopoietic transition contributes to pulmonary arterial hypertension.Cardiovasc Res. 2017 Nov 1;113(13):1560-1573. doi: 10.1093/cvr/cvx161. Cardiovasc Res. 2017. PMID: 29016733 Free PMC article.
-
[Earlier hematopoietic reconstitution by mouse cord blood transplantation combined with bone marrow c-kit(+) hematopoietic progenitor cells].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011 Apr;19(2):416-21. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011. PMID: 21518499 Chinese.
-
[Transplantation of mesenchymal derived stem cells followed by G-CSF injection can reconstitute hematopoiesis of lethally irradiated BALB/c mice].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002 Feb;24(1):20-4. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002. PMID: 12905834 Chinese.
-
Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials.Pharmacol Ther. 2010 Apr;126(1):1-8. doi: 10.1016/j.pharmthera.2009.12.006. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117135 Review.
-
Endothelial progenitor cells in pulmonary arterial hypertension.Trends Cardiovasc Med. 2010 Jan;20(1):22-9. doi: 10.1016/j.tcm.2010.03.003. Trends Cardiovasc Med. 2010. PMID: 20685574 Review.
Cited by
-
Plasma Cell-Free DNA Predicts Survival and Maps Specific Sources of Injury in Pulmonary Arterial Hypertension.Circulation. 2022 Oct 4;146(14):1033-1045. doi: 10.1161/CIRCULATIONAHA.121.056719. Epub 2022 Aug 25. Circulation. 2022. PMID: 36004627 Free PMC article.
-
Inflammatory Macrophage Expansion in Pulmonary Hypertension Depends upon Mobilization of Blood-Borne Monocytes.J Immunol. 2018 May 15;200(10):3612-3625. doi: 10.4049/jimmunol.1701287. Epub 2018 Apr 9. J Immunol. 2018. PMID: 29632145 Free PMC article.
-
Adverse effects of BMPR2 suppression in macrophages in animal models of pulmonary hypertension.Pulm Circ. 2019 May 24;10(1):2045894019856483. doi: 10.1177/2045894019856483. Online ahead of print. Pulm Circ. 2019. PMID: 31124398 Free PMC article.
-
Teriparatide Treatment Improves Bone Defect Healing Via Anabolic Effects on New Bone Formation and Non-Anabolic Effects on Inhibition of Mast Cells in a Murine Cranial Window Model.J Bone Miner Res. 2017 Sep;32(9):1870-1883. doi: 10.1002/jbmr.3178. Epub 2017 Jul 19. J Bone Miner Res. 2017. PMID: 28556967 Free PMC article.
-
Endothelial to haematopoietic transition contributes to pulmonary arterial hypertension.Cardiovasc Res. 2017 Nov 1;113(13):1560-1573. doi: 10.1093/cvr/cvx161. Cardiovasc Res. 2017. PMID: 29016733 Free PMC article.
References
-
- Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow–derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2005;127:1793–1798. - PubMed
-
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. - PubMed
-
- Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

