Notch-Dependent Pituitary SOX2(+) Stem Cells Exhibit a Timed Functional Extinction in Regulation of the Postnatal Gland
- PMID: 26651607
- PMCID: PMC4682291
- DOI: 10.1016/j.stemcr.2015.11.001
Notch-Dependent Pituitary SOX2(+) Stem Cells Exhibit a Timed Functional Extinction in Regulation of the Postnatal Gland
Abstract
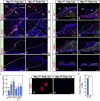
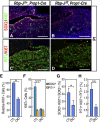
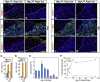
Although SOX2(+) stem cells are present in the postnatal pituitary gland, how they are regulated molecularly and whether they are required for pituitary functions remain unresolved questions. Using a conditional knockout animal model, here we demonstrate that ablation of the canonical Notch signaling in the embryonic pituitary gland leads to progressive depletion of the SOX2(+) stem cells and hypoplastic gland. Furthermore, we show that the SOX2(+) stem cells initially play a significant role in contributing to postnatal pituitary gland expansion by self-renewal and differentiating into distinct lineages in the immediate postnatal period. However, we found that within several weeks postpartum, the SOX2(+) stem cells switch to an essentially dormant state and are no longer required for homeostasis/tissue adaptation. Our results present a dynamic tissue homeostatic model in which stem cells provide an initial contribution to the growth of the neonatal pituitary gland, whereas the mature gland can be maintained in a stem cell-independent fashion.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential.Cell Stem Cell. 2013 Oct 3;13(4):433-45. doi: 10.1016/j.stem.2013.07.004. Cell Stem Cell. 2013. PMID: 24094324
-
Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland.Stem Cells Dev. 2012 Mar 20;21(5):801-13. doi: 10.1089/scd.2011.0496. Epub 2011 Nov 16. Stem Cells Dev. 2012. PMID: 21970375
-
Expression studies of neuronatin in prenatal and postnatal rat pituitary.Cell Tissue Res. 2016 May;364(2):273-88. doi: 10.1007/s00441-015-2325-2. Epub 2015 Nov 27. Cell Tissue Res. 2016. PMID: 26613603
-
Stem cells in the pituitary gland: A burgeoning field.Gen Comp Endocrinol. 2010 May 1;166(3):478-88. doi: 10.1016/j.ygcen.2009.11.007. Epub 2009 Nov 14. Gen Comp Endocrinol. 2010. PMID: 19917287 Review.
-
Pituitary stem cell regulation: who is pulling the strings?J Endocrinol. 2017 Sep;234(3):R135-R158. doi: 10.1530/JOE-17-0083. Epub 2017 Jun 14. J Endocrinol. 2017. PMID: 28615294 Review.
Cited by
-
Primary cilia and BBS4 are required for postnatal pituitary development.bioRxiv [Preprint]. 2025 Jul 18:2025.07.15.664994. doi: 10.1101/2025.07.15.664994. bioRxiv. 2025. PMID: 40791458 Free PMC article. Preprint.
-
Pituitary Remodeling Throughout Life: Are Resident Stem Cells Involved?Front Endocrinol (Lausanne). 2021 Jan 29;11:604519. doi: 10.3389/fendo.2020.604519. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33584539 Free PMC article. Review.
-
Pituitary stem cells: past, present and future perspectives.Nat Rev Endocrinol. 2024 Feb;20(2):77-92. doi: 10.1038/s41574-023-00922-4. Epub 2023 Dec 15. Nat Rev Endocrinol. 2024. PMID: 38102391 Free PMC article. Review.
-
Single-cell transcriptomic analysis of adult mouse pituitary reveals sexual dimorphism and physiologic demand-induced cellular plasticity.Protein Cell. 2020 Aug;11(8):565-583. doi: 10.1007/s13238-020-00705-x. Epub 2020 Mar 19. Protein Cell. 2020. PMID: 32193873 Free PMC article.
-
Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary.Int J Mol Sci. 2016 Jan 9;17(1):75. doi: 10.3390/ijms17010075. Int J Mol Sci. 2016. PMID: 26761002 Free PMC article. Review.
References
-
- Andoniadou C.L., Matsushima D., Mousavy Gharavy S.N., Signore M., Mackintosh A.I., Schaeffer M., Gaston-Massuet C., Mollard P., Jacques T.S., Le Tissier P. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. - PubMed
-
- Carbajo-Pérez E., Watanabe Y.G. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

