The domestic cat as a natural animal model of Alzheimer's disease
- PMID: 26651821
- PMCID: PMC4674944
- DOI: 10.1186/s40478-015-0258-3
The domestic cat as a natural animal model of Alzheimer's disease
Abstract
Introduction: Alzheimer's disease (AD) is the most dominant neurodegenerative disorder that causes dementia, and no effective treatments are available. To study its pathogenesis and develop therapeutics, animal models representing its pathologies are needed. Although many animal species develop senile plaques (SP) composed of amyloid-β (Aβ) proteins that are identical to those found in humans, none of them exhibit neurofibrillary tangles (NFT) and subsequent neurodegeneration, which are integral parts of the pathology of AD.
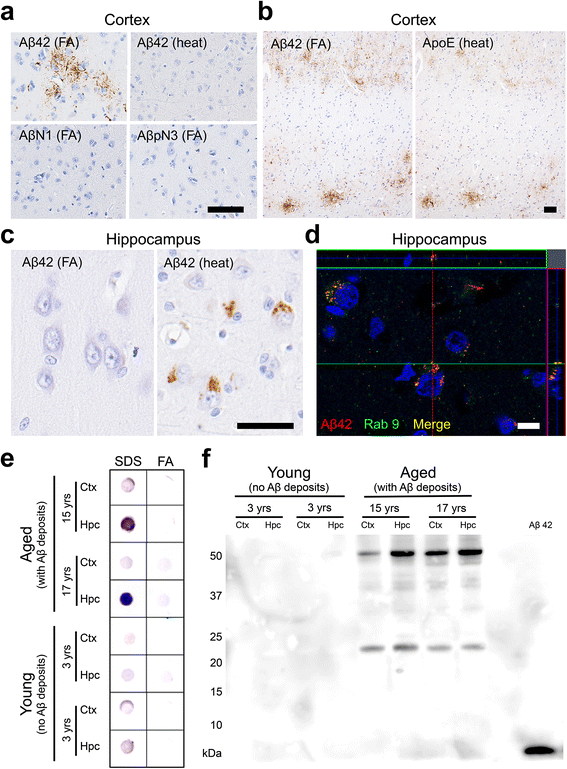
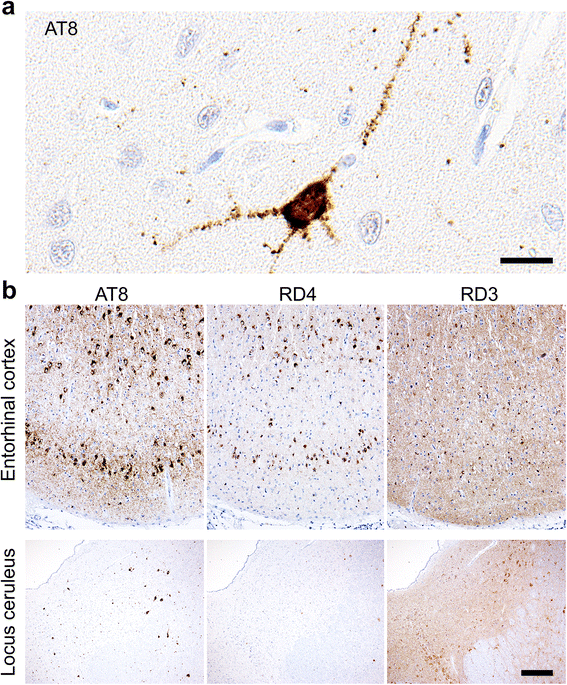
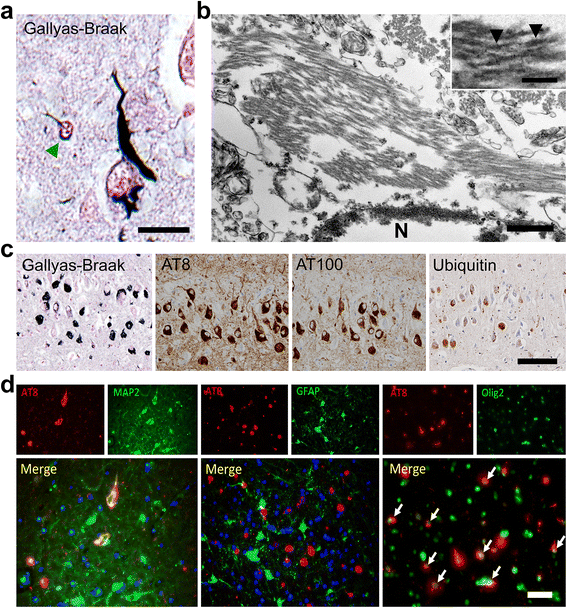
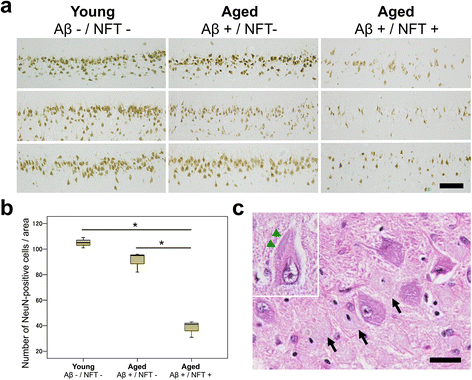
Results: The present study shows that Aβ accumulation, NFT formation, and significant neuronal loss all emerge naturally in the hippocampi of aged domestic cats. The NFT that form in the cat brain are identical to those seen in human AD in terms of their spatial distribution, the cells they affect, and the tau isoforms that comprise them. Interestingly, aged cats do not develop mature argyrophilic SP, but instead accumulate intraneuronal Aβ oligomers in their hippocampal pyramidal cells, which might be due to the amino acid sequence of felid Aβ.
Conclusions: These results suggest that Aβ oligomers are more important than SP for NFT formation and the subsequent neurodegeneration. The domestic cat is a unique animal species that naturally replicates various AD pathologies, especially Aβ oligomer accumulation, NFT formation, and neuronal loss.
Figures


References
-
- Aho L, Pikkarainen M, Hiltunen M, Leinonen V, Alafuzoff I. Immunohistochemical visualization of amyloid-beta protein precursor and amyloid-beta in extra- and intracellular compartments in the human brain. J Alzheimers Dis. 2010;20:1015–28. - PubMed
-
- Brinkmalm G, Portelius E, Öhrfelt A, Mattsson N, Persson R, Gustavsson MK, et al. An online nano-LC-ESI-FTICR-MS method for comprehensive characterization of endogenous fragments from amyloid β and amyloid precursor protein in human and cat cerebrospinal fluid. J Mass Spectrom. 2012;47:591–603. doi: 10.1002/jms.2987. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

