Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis
- PMID: 26652292
- PMCID: PMC4674993
- DOI: 10.1186/s13287-015-0231-x
Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis
Abstract
Introduction: Mesenchymal stem cells (MSCs) have been identified as a viable treatment for inflammatory bowel disease (IBD). MSCs derived from bone marrow (BM-MSCs) have predominated in experimental models whereas the majority of clinical trials have used MSCs derived from adipose tissue (AT-MSCs), thus there is little consensus on the optimal tissue source. The therapeutic efficacies of these MSCs are yet to be compared in context of the underlying dysfunction of the enteric nervous system innervating the gastrointestinal tract concomitant with IBD. This study aims to characterise the in vitro properties of MSCs and compare their in vivo therapeutic potential for the treatment of enteric neuropathy associated with intestinal inflammation.
Methods: BM-MSCs and AT-MSCs were validated and characterised in vitro. In in vivo experiments, guinea-pigs received either 2,4,6-trinitrobenzene-sulfonate acid (TNBS) for the induction of colitis or sham treatment by enema. MSCs were administered at a dose of 1x10(6) cells via enema 3 hours after the induction of colitis. Colon tissues were collected 24 and 72 hours after TNBS administration to assess the level of inflammation and damage to the ENS. MSC migration to the myenteric plexus in vivo was elucidated by immunohistochemistry and in vitro using a modified Boyden chamber assay.
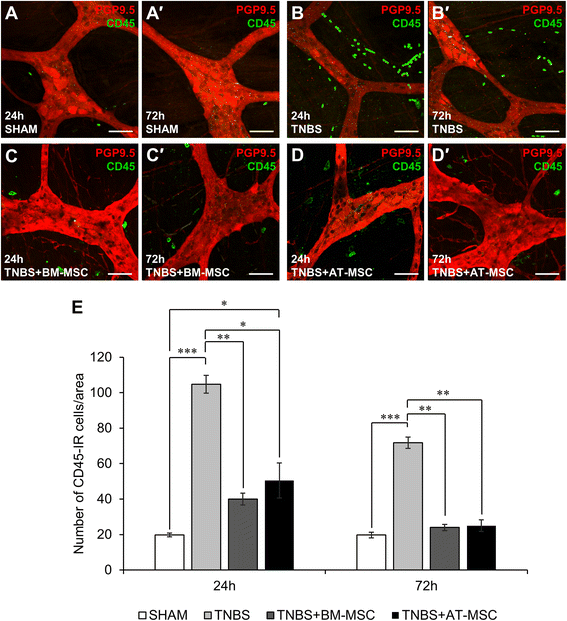
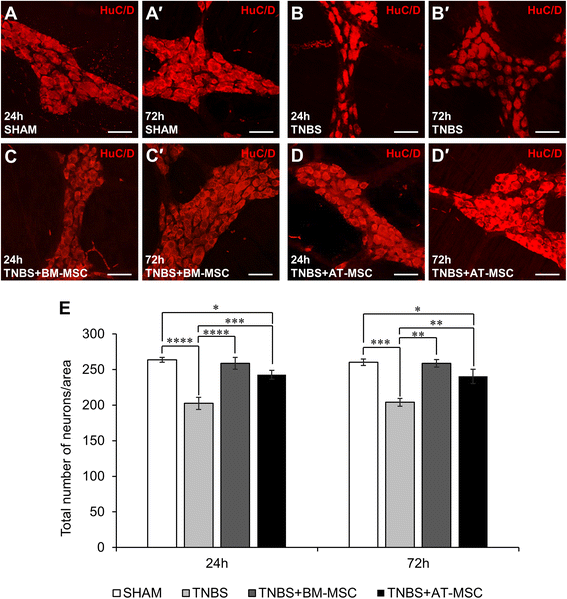
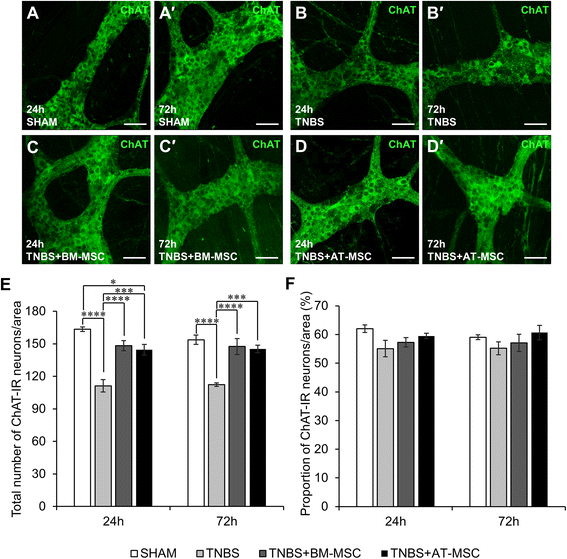
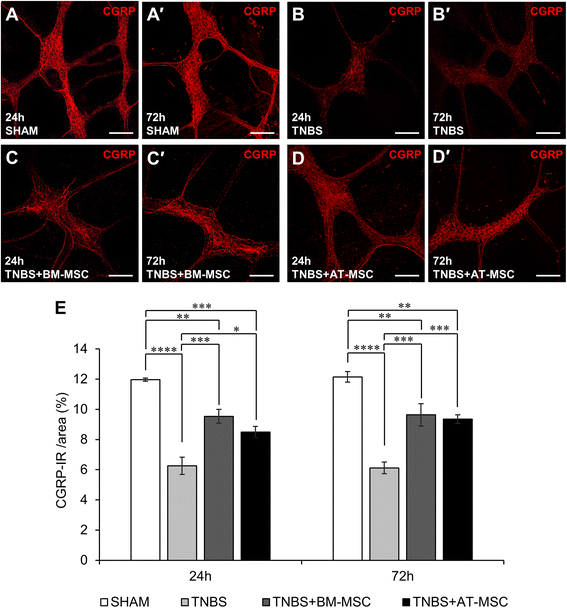
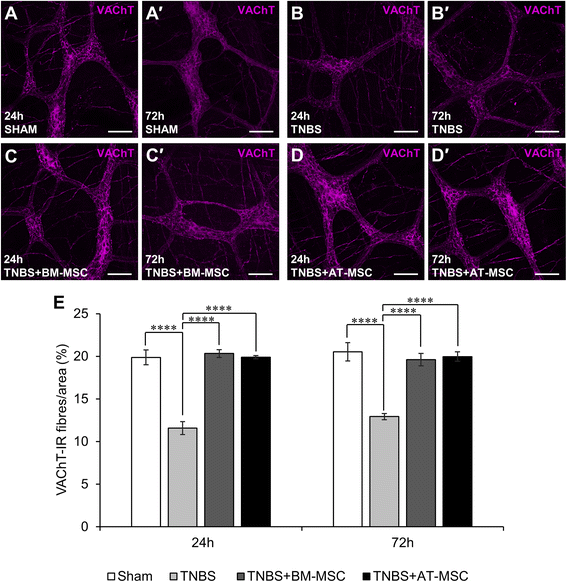
Results: Cells exhibited multipotency and a typical surface immunophenotype for validation as bona fide MSCs. In vitro characterisation revealed distinct differences in growth kinetics, clonogenicity and cell morphology between MSC types. In vivo, BM-MSCs were comparatively more effective than AT-MSCs in attenuating leukocyte infiltration and neuronal loss in the myenteric plexus. MSCs from both sources equally ameliorated body weight loss, gross morphological damage to the colon, changes in the neurochemical coding of neuronal subpopulations and the reduction in density of extrinsic and intrinsic nerve fibres innervating the colon. MSCs from both sources migrated to the myenteric plexus in in vivo colitis and in an in vitro assay.
Conclusions: These data from in vitro experiments suggest that AT-MSCs are ideal for cellular expansion. However, BM-MSCs were more therapeutic in the treatment of enteric neuropathy and plexitis. These characteristics should be considered when deciding on the MSC tissue source.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

