Assessing kinetic and epitopic diversity across orthogonal monoclonal antibody generation platforms
- PMID: 26652308
- PMCID: PMC4966639
- DOI: 10.1080/19420862.2015.1118596
Assessing kinetic and epitopic diversity across orthogonal monoclonal antibody generation platforms
Abstract
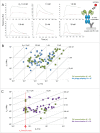
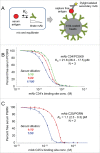
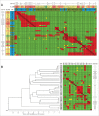
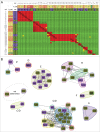
The ability of monoclonal antibodies (mAbs) to target specific antigens with high precision has led to an increasing demand to generate them for therapeutic use in many disease areas. Historically, the discovery of therapeutic mAbs has relied upon the immunization of mammals and various in vitro display technologies. While the routine immunization of rodents yields clones that are stable in serum and have been selected against vast arrays of endogenous, non-target self-antigens, it is often difficult to obtain species cross-reactive mAbs owing to the generally high sequence similarity shared across human antigens and their mammalian orthologs. In vitro display technologies bypass this limitation, but lack an in vivo screening mechanism, and thus may potentially generate mAbs with undesirable binding specificity and stability issues. Chicken immunization is emerging as an attractive mAb discovery method because it combines the benefits of both in vivo and in vitro display methods. Since chickens are phylogenetically separated from mammals, their proteins share less sequence homology with those of humans, so human proteins are often immunogenic and can readily elicit rodent cross-reactive clones, which are necessary for in vivo proof of mechanism studies. Here, we compare the binding characteristics of mAbs isolated from chicken immunization, mouse immunization, and phage display of human antibody libraries. Our results show that chicken-derived mAbs not only recapitulate the kinetic diversity of mAbs sourced from other methods, but appear to offer an expanded repertoire of epitopes. Further, chicken-derived mAbs can bind their native serum antigen with very high affinity, highlighting their therapeutic potential.
Keywords: Binning; Immunology; chicken immune repertoire; epitope; monoclonal antibody.
Figures
Similar articles
-
Sym021, a promising anti-PD1 clinical candidate antibody derived from a new chicken antibody discovery platform.MAbs. 2019 May/Jun;11(4):666-680. doi: 10.1080/19420862.2019.1596514. Epub 2019 May 3. MAbs. 2019. PMID: 31046547 Free PMC article. Clinical Trial.
-
Chickens with humanized immunoglobulin genes generate antibodies with high affinity and broad epitope coverage to conserved targets.MAbs. 2018 Jan;10(1):71-80. doi: 10.1080/19420862.2017.1386825. Epub 2017 Nov 2. MAbs. 2018. PMID: 29035625 Free PMC article.
-
Phage display and hybridoma generation of antibodies to human CXCR2 yields antibodies with distinct mechanisms and epitopes.MAbs. 2014;6(6):1425-38. doi: 10.4161/mabs.34376. MAbs. 2014. PMID: 25484064 Free PMC article.
-
Phage antibody display libraries: a powerful antibody discovery platform for immunotherapy.Crit Rev Biotechnol. 2016;36(2):276-89. doi: 10.3109/07388551.2014.958978. Epub 2014 Nov 14. Crit Rev Biotechnol. 2016. PMID: 25394539 Review.
-
The emerging role of biosensor-based epitope binning and mapping in antibody-based drug discovery.Expert Opin Drug Discov. 2016 Oct;11(10):925-37. doi: 10.1080/17460441.2016.1229295. Epub 2016 Sep 8. Expert Opin Drug Discov. 2016. PMID: 27600831 Review.
Cited by
-
Characterizing Epitope Binding Regions of Entire Antibody Panels by Combining Experimental and Computational Analysis of Antibody: Antigen Binding Competition.Molecules. 2020 Aug 11;25(16):3659. doi: 10.3390/molecules25163659. Molecules. 2020. PMID: 32796656 Free PMC article.
-
Point Mutations in Retargeted gD Eliminate the Sensitivity of EGFR/EGFRvIII-Targeted HSV to Key Neutralizing Antibodies.Mol Ther Methods Clin Dev. 2020 Jan 13;16:145-154. doi: 10.1016/j.omtm.2019.12.013. eCollection 2020 Mar 13. Mol Ther Methods Clin Dev. 2020. PMID: 32042851 Free PMC article.
-
Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives.Int Immunopharmacol. 2020 Aug;85:106639. doi: 10.1016/j.intimp.2020.106639. Epub 2020 May 27. Int Immunopharmacol. 2020. PMID: 32473573 Free PMC article. Review.
-
Antibodies Targeting Closely Adjacent or Minimally Overlapping Epitopes Can Displace One Another.PLoS One. 2017 Jan 6;12(1):e0169535. doi: 10.1371/journal.pone.0169535. eCollection 2017. PLoS One. 2017. PMID: 28060885 Free PMC article.
-
Broad epitope coverage of a human in vitro antibody library.MAbs. 2017 Jan;9(1):29-42. doi: 10.1080/19420862.2016.1246096. Epub 2016 Oct 17. MAbs. 2017. PMID: 27748644 Free PMC article.
References
-
- Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep 2015 Jun 12; 35(4). pii: e00225; PMID: 26182432; http://dx.doi.org/10.1042/BSR20150089 - DOI - PMC - PubMed
-
- Shekarian T, Valsesia-Wittmann S, Caux C, Marabelle A. Paradigm shift in oncology: targeting the immune system rather than cancer cells. Mutagenesis 2015 Mar; 30(2):205–11; PMID: 25688113; http://dx.doi.org/10.1093/mutage/geu073 - DOI - PubMed
-
- Ascierto ML, Melero I, Ascierto PA Melanoma: From Incurable Beast to a Curable Bet. The Success of Immunotherapy. Front Oncol 2015 Jul 13; 5:152; PMID: 26217587; http://dx.doi.org/10.3389/fonc.2015.00152 - DOI - PMC - PubMed
-
- Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol 1999 Jun; 17(6):568–72; PMID: 10385321; http://dx.doi.org/10.1038/9872 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
