HisAK70: progress towards a vaccine against different forms of leishmaniosis
- PMID: 26653170
- PMCID: PMC4675018
- DOI: 10.1186/s13071-015-1246-y
HisAK70: progress towards a vaccine against different forms of leishmaniosis
Abstract
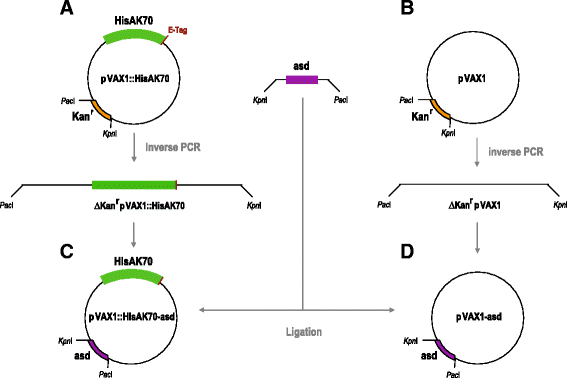
Background: Leishmania major and Leishmania infantum are among the main species that are responsible for cutaneous leishmaniosis (CL) and visceral leishmaniosis (VL), respectively. The leishmanioses represent the second-largest parasitic killer in the world after malaria. Recently, we succeeded in generating a plasmid DNA (pCMV-HISA70m2A) and demonstrated that immunized mice were protected against L. major challenge. The efficacy of the DNA-vaccine was further enhanced by the inclusion of KMP-11 antigen into the antibiotic-free plasmid pVAX1-asd.
Methods: Here, we describe the use of a HisAK70 DNA-vaccine encoding seven Leishmania genes (H2A, H2B, H3, H4, A2, KMP11 and HSP70) for vaccination of mice to assess the induction of a resistant phenotype against VL and CL.
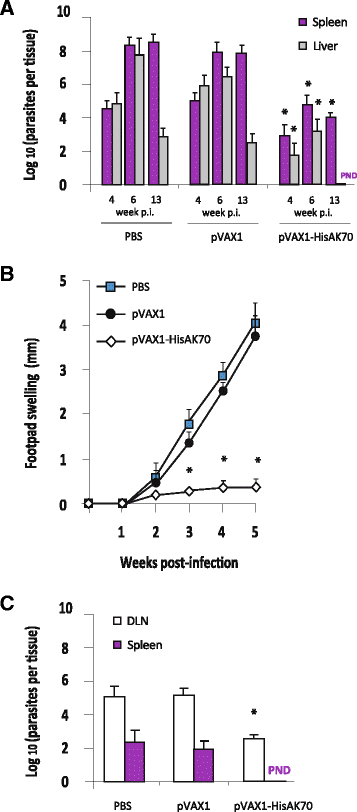
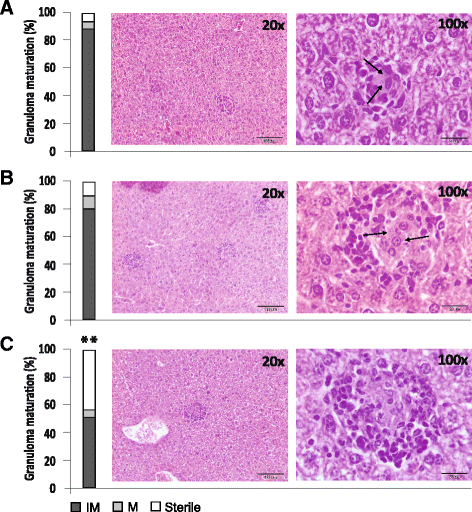
Results: HisAK70 was successful in vaccinated mice, resulting in a high amount of efficient sterile hepatic granulomas associated with a hepatic parasite burden fully resolved in the VL model; and resulting in 100% inhibition of parasite visceralization in the CL model.
Conclusions: The results suggest that immunization with the HisAK70 DNA-vaccine may provide a rapid, suitable, and efficient vaccination strategy to confer cross-protective immunity against VL and CL.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

