Characteristics of Alpha-1 Antitrypsin-Deficient Individuals in the Long-term Oxygen Treatment Trial and Comparison with Other Subjects with Chronic Obstructive Pulmonary Disease
- PMID: 26653189
- PMCID: PMC4722829
- DOI: 10.1513/AnnalsATS.201506-389OC
Characteristics of Alpha-1 Antitrypsin-Deficient Individuals in the Long-term Oxygen Treatment Trial and Comparison with Other Subjects with Chronic Obstructive Pulmonary Disease
Abstract
Rationale: Alpha-1 antitrypsin deficiency (AATD) predisposes to chronic obstructive pulmonary disease, but is underrecognized. Oxygenation and exercise desaturation in individuals with AATD-associated chronic obstructive pulmonary disease has been sparsely studied. The Long-term Oxygen Treatment Trial (LOTT) permits comparing these features of individuals with AATD with alpha-1 antitrypsin-replete (called "usual chronic obstructive pulmonary disease") LOTT participants.
Objectives: Compare demographic, clinical, baseline oxygenation, and exercise desaturation features in participating AATD subjects with those of other LOTT subjects.
Methods: LOTT is a multicenter randomized controlled trial comparing use of supplemental oxygen versus not in subjects with chronic obstructive pulmonary disease and moderate hypoxemia (resting oxygen saturation as measured by pulse oximetry, 89-93%) or normal oxygen saturation at rest and significant exercise desaturation.
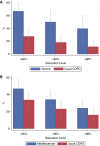
Measurement and main results: Among the 597 LOTT participants with nonmissing alpha-1 antitrypsin levels, 11 (1.8%) had severe AATD and 44 (7.4%) had mild/moderate AATD. Comparison of the 11 severely AAT-deficient individuals with the 542 LOTT participants with usual chronic obstructive pulmonary disease showed that the AATD subjects were younger and despite less smoking, had lower FEV1/FVC (mean post-bronchodilator FEV1/FVC, 0.38 ± 0.06 vs. 0.46 ± 0.13; P = 0.002). Comparison with 27 age-, sex-, and FEV1-matched alpha-1 antitrypsin-normal LOTT participants showed no baseline difference in resting room air pulse oximetry saturation (AATD, 93.6% ± 2.3% vs. 92.7% ± 2.2%; P = 0.64). Exercise-related desaturation was more severe in the individuals with AATD based on desaturation to 88% or less sooner during a 6-minute-walk test, having a higher percentage of desaturation points (e.g., <90%) during exercise, and having a higher distance-saturation product (defined as the distance walked in 6 min multiplied by the nadir saturation achieved during the 6-minute-walk test).
Conclusions: These data suggest that individuals with AATD experience more profound desaturation with exercise than age-, sex-, race-, and FEV1-matched control subjects with usual chronic obstructive pulmonary disease. Clinical trial registered with www.clinicaltrials.gov (NCT 00692198).
Trial registration: ClinicalTrials.gov NCT00692198.
Keywords: Long-term Oxygen Treatment Trial; alpha-1 antitrypsin deficiency; desaturation; oxygenation.
Figures
References
-
- Taliercio RM, Chatburn RL, Stoller JK. Knowledge of alpha-1 antitrypsin deficiency among internal medicine house officers and respiratory therapists: results of a survey. Respir Care. 2010;55:322–327. - PubMed
-
- Greulich T, Ottaviani S, Bals R, Lepper PM, Vogelmeier C, Luisetti M, Ferrarotti I. Alpha1-antitrypsin deficiency: diagnostic testing and disease awareness in Germany and Italy. Respir Med. 2013;107:1400–1408. - PubMed
-
- Stoller JK, Smith P, Yang P, Spray J. Physical and social impact of alpha 1-antitrypsin deficiency: results of a survey. Cleve Clin J Med. 1994;61:461–467. - PubMed
-
- Stoller JK, Sandhaus RA, Turino G, Dickson R, Rodgers K, Strange C. Delay in diagnosis of alpha1-antitrypsin deficiency: a continuing problem. Chest. 2005;128:1989–1994. - PubMed
-
- Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128:1179–1186. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN268200736197C/PHS HHS/United States
- HHSN268200736191C/PHS HHS/United States
- HHSN268200736196C/PHS HHS/United States
- HHSN268200736184C/PHS HHS/United States
- HHSN268200736189C/PHS HHS/United States
- Y1-HR-8076-01/HR/NHLBI NIH HHS/United States
- HHSN268200736183C/PHS HHS/United States
- HHSN268200736190C/PHS HHS/United States
- HHSN268200736186C/PHS HHS/United States
- HHSN268200736192C/PHS HHS/United States
- Y1-HR-7019-01/HR/NHLBI NIH HHS/United States
- HHSN268200736195C/PHS HHS/United States
- HHSN268200736193C/PHS HHS/United States
- HHSN268200736187C/PHS HHS/United States
- HHSN268200736185C/PHS HHS/United States
- HHSN268200736194C/PHS HHS/United States
- HHSN268200736188C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

