Simultaneous inhibition of JAK and SYK kinases ameliorates chronic and destructive arthritis in mice
- PMID: 26653844
- PMCID: PMC4675041
- DOI: 10.1186/s13075-015-0866-0
Simultaneous inhibition of JAK and SYK kinases ameliorates chronic and destructive arthritis in mice
Abstract
Introduction: Despite the broad spectrum of antirheumatic drugs, RA is still not well controlled in up to 30-50 % of patients. Inhibition of JAK kinases by means of the pan-JAK inhibitor tofacitinib has demonstrated to be effective even in difficult-to-treat patients. Here, we discuss whether the efficacy of JAK inhibition can be improved by simultaneously inhibiting SYK kinase, since both kinases mediate complementary and non-redundant pathways in RA.
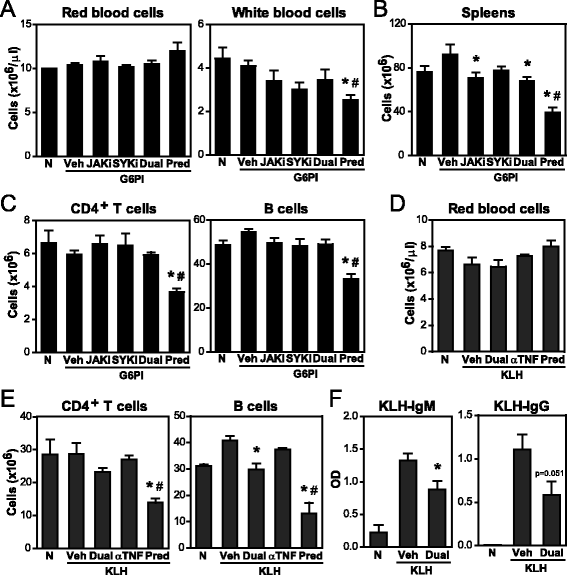
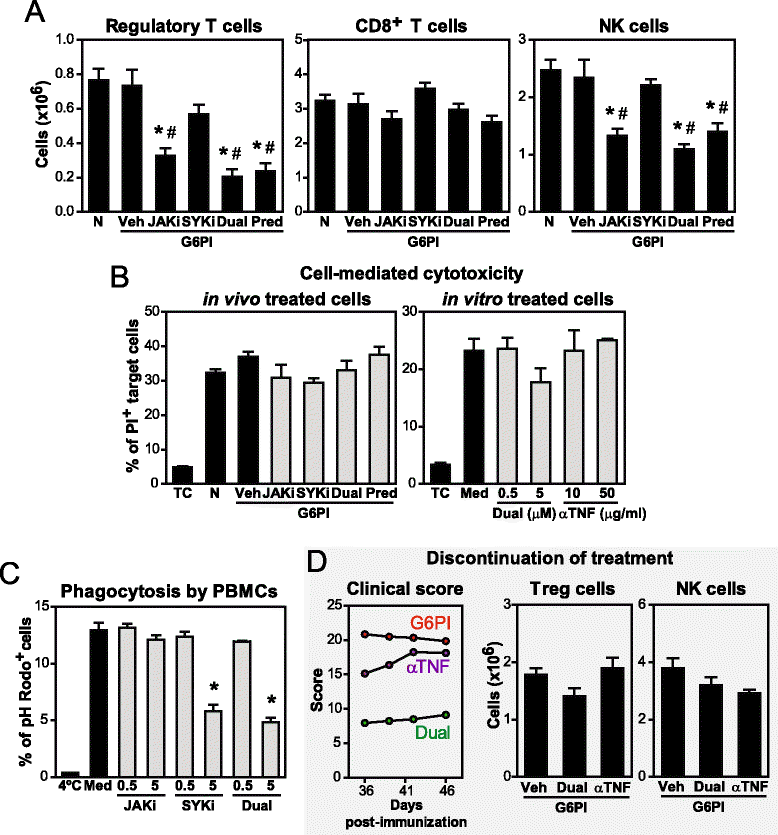
Methods: Efficacy of dual JAK + SYK inhibition with selective small molecule inhibitors was evaluated in chronic G6PI-induced arthritis, a non-self-remitting and destructive arthritis model in mice. Clinical and histopathological scores, as well as cytokine and anti-G6PI antibody production were assessed in both preventive and curative protocols. Potential immunotoxicity was also evaluated in G6PI-induced arthritis and in a 28-day TDAR model, by analysing the effects of JAK + SYK inhibition on hematological parameters, lymphoid organs, leukocyte subsets and cell function.
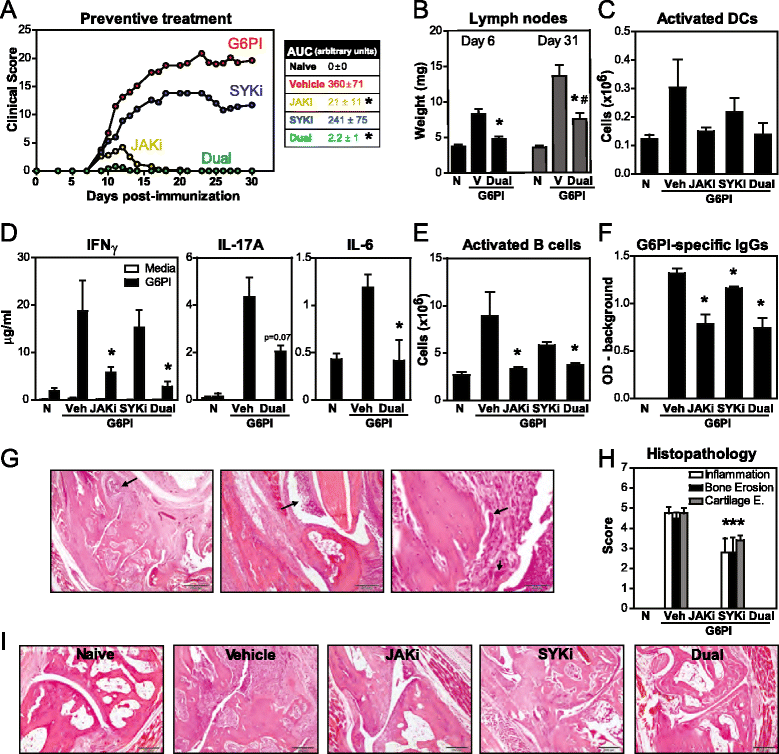
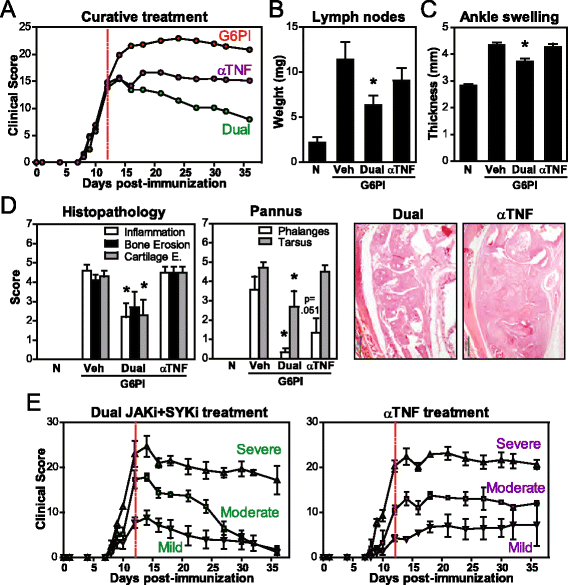
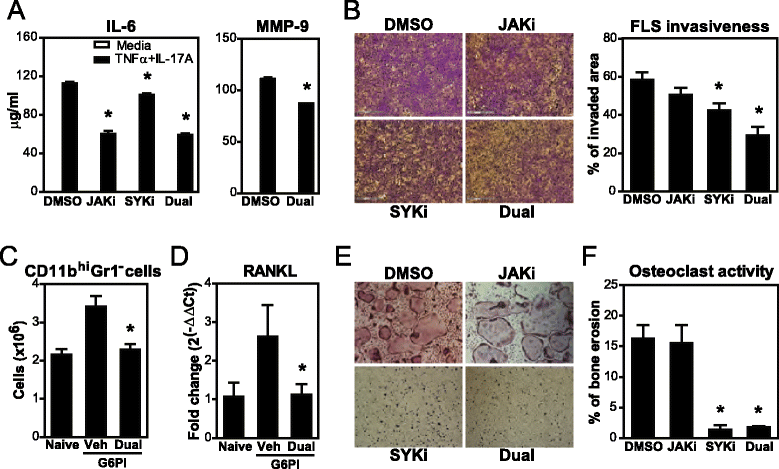
Results: Simultaneous JAK + SYK inhibition completely prevented mice from developing arthritis. This therapeutic strategy was also very effective in ameliorating already established arthritis. Dual kinase inhibition immediately resulted in greatly decreased clinical and histopathological scores and led to disease remission in over 70 % of the animals. In contrast, single JAK inhibition and anti-TNF therapy (etanercept) were able to stop disease progression but not to revert it. Dual kinase inhibition decreased Treg and NK cell counts to the same extent as single JAK inhibition but overall cytotoxicity remained intact. Interestingly, treatment discontinuation rapidly reversed such immune cell reduction without compromising clinical efficacy, suggesting long-lasting curative effects. Dual kinase inhibition reduced the Th1/Th17 cytokine cascade and the differentiation and function of joint cells, in particular osteoclasts and fibroblast-like synoviocytes.
Conclusions: Concurrent JAK + SYK inhibition resulted in higher efficacy than single kinase inhibition and TNF blockade in a chronic and severe arthritis model. Thus, blockade of multiple immune signals with dual JAK + SYK inhibition represents a reasonable therapeutic strategy for RA, in particular in patients with inadequate responses to current treatments. Our data supports the multiplicity of events underlying this heterogeneous and complex disease.
Figures


References
-
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

