Protein flexibility is required for vesicle tethering at the Golgi
- PMID: 26653856
- PMCID: PMC4721967
- DOI: 10.7554/eLife.12790
Protein flexibility is required for vesicle tethering at the Golgi
Abstract
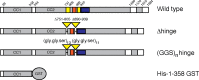
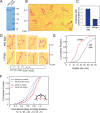
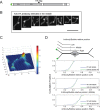
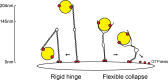
The Golgi is decorated with coiled-coil proteins that may extend long distances to help vesicles find their targets. GCC185 is a trans Golgi-associated protein that captures vesicles inbound from late endosomes. Although predicted to be relatively rigid and highly extended, we show that flexibility in a central region is required for GCC185’s ability to function in a vesicle tethering cycle. Proximity ligation experiments show that that GCC185’s N-and C-termini are within <40 nm of each other on the Golgi. In physiological buffers without fixatives, atomic force microscopy reveals that GCC185 is shorter than predicted, and its flexibility is due to a central bubble that represents local unwinding of specific sequences. Moreover, 85% of the N-termini are splayed, and the splayed N-terminus can capture transport vesicles in vitro. These unexpected features support a model in which GCC185 collapses onto the Golgi surface, perhaps by binding to Rab GTPases, to mediate vesicle tethering.
Keywords: Golgi complex; biochemistry; cell biology; coiled-coil; human; membrane traffic; vesicle tethering.
Conflict of interest statement
SRP: Reviewing editor,
The other authors declare that no competing interests exist.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

