Shared and Distinct Mechanisms of Compartmentalized and Cytosolic Ciliogenesis
- PMID: 26654377
- PMCID: PMC5857621
- DOI: 10.1016/j.cub.2015.11.001
Shared and Distinct Mechanisms of Compartmentalized and Cytosolic Ciliogenesis
Abstract
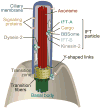
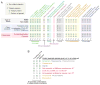
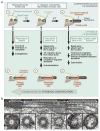
Most motile and all non-motile (also known as primary) eukaryotic cilia possess microtubule-based axonemes that are assembled at the cell surface to form hair-like or more elaborate compartments endowed with motility and/or signaling functions. Such compartmentalized ciliogenesis depends on the core intraflagellar transport (IFT) machinery and the associated Bardet-Biedl syndrome complex (BBSome) for dynamic delivery of ciliary components. The transition zone (TZ), an ultrastructurally complex barrier or 'gate' at the base of cilia, also contributes to the formation of compartmentalized cilia. Yet, some ciliated protists do not have IFT components and, like some metazoan spermatozoa, use IFT-independent mechanisms to build axonemes exposed to the cytosol. Moreover, various ciliated protists lack TZ components, whereas Drosophila sperm surprisingly requires the activity of dynamically localized TZ proteins for cytosolic ciliogenesis. Here, we discuss the various ways eukaryotes use IFT and/or TZ proteins to generate the wide assortment of compartmentalized and cytosolic cilia observed in nature. Consideration of the different ciliogenesis pathways allows us to propose how three types of cytosol-exposed cilia (primary, secondary and tertiary), including cilia found in the human sperm proximal segment, are likely generated by evolutionary derivations of compartmentalized ciliogenesis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. - PubMed
-
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. - PubMed
-
- Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123(Pt 4):505–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

