Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort
- PMID: 26654443
- PMCID: PMC4675035
- DOI: 10.1186/s40360-015-0036-7
Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort
Abstract
Background: Amikacin and kanamycin are mainly used for treating multidrug-resistant tuberculosis (MDR-TB), especially in developing countries where the burden of MDR-TB is highest. Their protracted use in MDR-TB treatment is known to cause dose-dependent irreversible hearing loss, requiring hearing aids, cochlear implants or rehabilitation. Therapeutic drug monitoring and regular audiological assessments may help to prevent or detect the onset of hearing loss, but these services are not always available or affordable in many developing countries. We aimed to compare the cumulative incidence of hearing loss among patients treated for MDR-TB with amikacin or kanamycin-based regimens, and to identify the most-at-risk patients, based on the real-life clinical practice experiences in Namibia.
Methods: We conducted a retrospective cohort study of patients treated with amikacin or kanamycin-based regimens in four public sector MDR-TB treatment sites in Namibia between June 2004 and March 2014. Patients were audiologically assessed as part of clinical care. The study outcome was the occurrence of any hearing loss. Data were manually extracted from patients' treatment records. We compared proportions using the Chi-square test; applied stratified analysis and logistic regression to study the risk of hearing loss and to identify the most-at-risk patients through effect-modification analysis. A P-value < 0.05 was statistically significant.
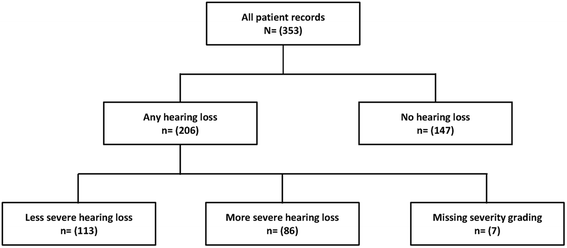
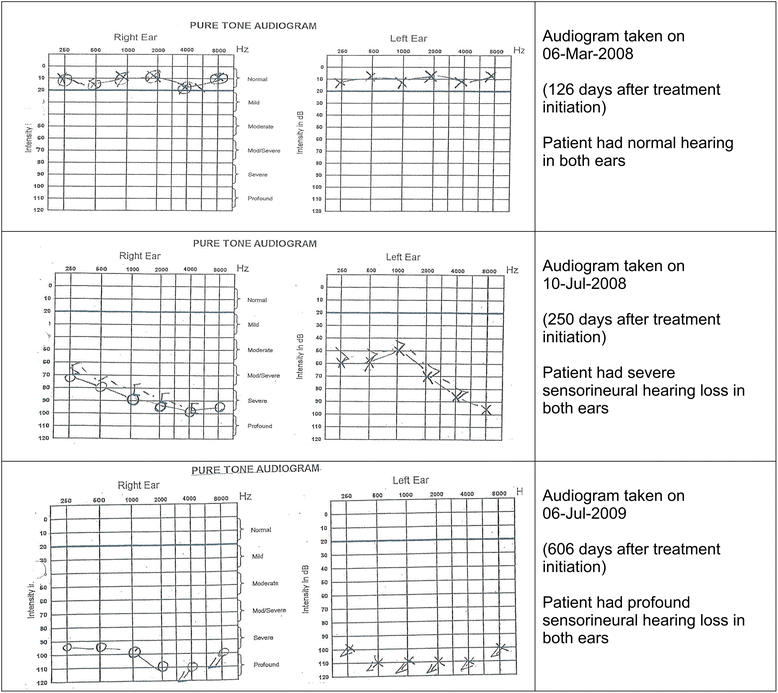
Results: All 353 patients had normal baseline hearing, 46 % were HIV co-infected. Cumulative incidence of any hearing loss was 58 %, which was mostly bilateral (83 %), and mild (32 %), moderate (23 %), moderate-severe (16 %), severe (10 %), or profound (15 %). Patients using amikacin had a greater risk of developing the more severe forms of hearing loss than those using kanamycin (adjusted odds ratio (OR) = 4.0, 95 % CI: 1.5-10.8). Patients co-infected with HIV (OR = 3.4, 95 % CI: 1.1-10.6), males (OR = 4.5, 95 %1.5-13.4) and those with lower baseline body weight (40-59 kg, OR = 2.8, 95 % CI: 1.1-6.8), were most-at-risk of developing hearing loss.
Conclusion: Amikacin use in the long-term MDR-TB treatment led to a higher risk of occurrence of the more severe forms of hearing loss compared to kanamycin use. Males, patients with low baseline body weight and those co-infected with HIV were most-at-risk. MDR-TB treatment programmes should consider replacing amikacin with kanamycin and strengthen the routine renal, serum therapeutic drug levels and audiometric monitoring in the most-at-risk patients treated with aminoglycosides.
Figures
Similar articles
-
Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection.Int J Tuberc Lung Dis. 2018 Jun 1;22(6):667-674. doi: 10.5588/ijtld.17.0830. Int J Tuberc Lung Dis. 2018. PMID: 29862952 Free PMC article. Review.
-
Reduced Chance of Hearing Loss Associated with Therapeutic Drug Monitoring of Aminoglycosides in the Treatment of Multidrug-Resistant Tuberculosis.Antimicrob Agents Chemother. 2017 Feb 23;61(3):e01400-16. doi: 10.1128/AAC.01400-16. Print 2017 Mar. Antimicrob Agents Chemother. 2017. PMID: 28069654 Free PMC article.
-
The importance of audiometric monitoring in patients with multidrug-resistant tuberculosis.Rev Soc Bras Med Trop. 2017 Sep-Oct;50(5):646-651. doi: 10.1590/0037-8682-0465-2016. Rev Soc Bras Med Trop. 2017. PMID: 29160511
-
Successful MDR-TB treatment regimens including amikacin are associated with high rates of hearing loss.BMC Infect Dis. 2014 Oct 9;14:542. doi: 10.1186/1471-2334-14-542. BMC Infect Dis. 2014. PMID: 25300708 Free PMC article.
-
The safety and tolerability of the second-line injectable antituberculosis drugs in children.Expert Opin Drug Saf. 2016 Nov;15(11):1491-1500. doi: 10.1080/14740338.2016.1223623. Epub 2016 Aug 22. Expert Opin Drug Saf. 2016. PMID: 27548570 Review.
Cited by
-
Case Report: Kanamycin Ototoxicity and MDR-TB Treatment Regimen.Int Med Case Rep J. 2021 Nov 26;14:815-817. doi: 10.2147/IMCRJ.S336259. eCollection 2021. Int Med Case Rep J. 2021. PMID: 34858067 Free PMC article.
-
Drug-Induced Ototoxicity: Diagnosis and Monitoring.Drug Saf. 2018 May;41(5):451-464. doi: 10.1007/s40264-017-0629-8. Drug Saf. 2018. PMID: 29404977 Review.
-
Safety and effectiveness of low-dose amikacin in nontuberculous mycobacterial pulmonary disease treated in Toronto, Canada.BMC Pharmacol Toxicol. 2019 Jun 3;20(1):37. doi: 10.1186/s40360-019-0302-1. BMC Pharmacol Toxicol. 2019. PMID: 31159865 Free PMC article.
-
Risk factors associated with post-tuberculosis sequelae: a systematic review and meta-analysis.EClinicalMedicine. 2024 Oct 21;77:102898. doi: 10.1016/j.eclinm.2024.102898. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39502524 Free PMC article.
-
Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection.Int J Tuberc Lung Dis. 2018 Jun 1;22(6):667-674. doi: 10.5588/ijtld.17.0830. Int J Tuberc Lung Dis. 2018. PMID: 29862952 Free PMC article. Review.
References
-
- World Health Organization (WHO) Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: Switzerland; 2008. - PubMed
-
- Ministry of Health and Social Services (MoHSS) National Guidelines for the Management of Tuberculosis. 3. Windhoek: MoHSS; 2012.
-
- Cianfrone G, Pentangelo D, Altissimi G, Turchetta R. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. 2011;15:601–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical