Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation
- PMID: 26655221
- PMCID: PMC4751396
- DOI: 10.1074/jbc.M115.678995
Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation
Abstract
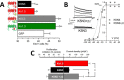
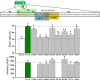
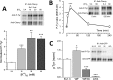
Changes in voltage-dependent potassium channels (Kv channels) associate to proliferation in many cell types, including transfected HEK293 cells. In this system Kv1.5 overexpression decreases proliferation, whereas Kv1.3 expression increases it independently of K(+) fluxes. To identify Kv1.3 domains involved in a proliferation-associated signaling mechanism(s), we constructed chimeric Kv1.3-Kv1.5 channels and point-mutant Kv1.3 channels, which were expressed as GFP- or cherry-fusion proteins. We studied their trafficking and functional expression, combining immunocytochemical and electrophysiological methods, and their impact on cell proliferation. We found that the C terminus is necessary for Kv1.3-induced proliferation. We distinguished two residues (Tyr-447 and Ser-459) whose mutation to alanine abolished proliferation. The insertion into Kv1.5 of a sequence comprising these two residues increased proliferation rate. Moreover, Kv1.3 voltage-dependent transitions from closed to open conformation induced MEK-ERK1/2-dependent Tyr-447 phosphorylation. We conclude that the mechanisms for Kv1.3-induced proliferation involve the accessibility of key docking sites at the C terminus. For one of these sites (Tyr-447) we demonstrated the contribution of MEK/ERK-dependent phosphorylation, which is regulated by voltage-induced conformational changes.
Keywords: Kv1.3; cell proliferation; electrophysiology; membrane potential; mutagenesis; potassium channel; structure-function; tyrosine phosphorylation; voltage-dependent conformation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Pardo L. A. (2004) Voltage-gated potassium channels in cell proliferation. Physiology 19, 285–292 - PubMed
-
- Coetzee W. A., Amarillo Y., Chiu J., Chow A., Lau D., McCormack T., Moreno H., Nadal M. S., Ozaita A., Pountney D., Saganich M., Vega-Saenz de Miera E., and Rudy B. (1999) Molecular diversity of K+ channels. Ann. N.Y. Acad. Sci. 868, 233–285 - PubMed
-
- DeCoursey T. E., Chandy K. G., Gupta S., and Cahalan M. D. (1984) Voltage-gated K+ channels in human lymphocyte-T: a role in mitogenesis. Nature 307, 465–468 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

