Dose-Response Analysis When There Is a Correlation between Affinity and Efficacy
- PMID: 26655305
- PMCID: PMC4746484
- DOI: 10.1124/mol.115.102509
Dose-Response Analysis When There Is a Correlation between Affinity and Efficacy
Abstract
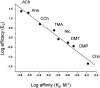
The shape of a concentration-response curve (CRC) is determined by underlying equilibrium constants for agonist binding and receptor conformational change. Typically, agonists are characterized by the empirical CRC parameters efficacy (the maximum response), EC(50) (the concentration that produces a half-maximum response), and the Hill coefficient (the maximum slope of the response). Ligands activate receptors because they bind with higher affinity to the active versus resting conformation, and in skeletal muscle nicotinic acetylcholine receptors there is an exponential relationship between these two equilibrium dissociation constants. Consequently, knowledge of two receptor-specific, agonist-independent constants--the activation equilibrium constant without agonists (E(0)) and the affinity-correlation exponent (M)--allows an entire CRC to be calculated from a measurement of either efficacy or affinity. I describe methods for estimating the CRCs of partial agonists in receptors that have a correlation between affinity and efficacy.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

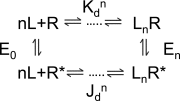

References
-
- Ashcroft FM. (2000) Ion channels and disease: channelopathies, Academic Press, San Diego.
-
- Changeux J-P, Edelstein SJ. (2005) Nicotinic acetylcholine receptors: from molecular biology to cognition. Odile Jacob, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

