Converging roles of caspases in inflammasome activation, cell death and innate immunity
- PMID: 26655628
- PMCID: PMC4915362
- DOI: 10.1038/nri.2015.7
Converging roles of caspases in inflammasome activation, cell death and innate immunity
Abstract
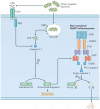
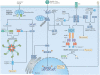
Inflammatory and apoptotic caspases are central players in inflammation and apoptosis, respectively. However, recent studies have revealed that these caspases have functions beyond their established roles. In addition to mediating cleavage of the inflammasome-associated cytokines interleukin-1β (IL-1β) and IL-18, inflammatory caspases modulate distinct forms of programmed cell death and coordinate cell-autonomous immunity and other fundamental cellular processes. Certain apoptotic caspases assemble structurally diverse and dynamic complexes that direct inflammasome and interferon responses to fine-tune inflammation. In this Review, we discuss the expanding and interconnected roles of caspases that highlight new aspects of this family of cysteine proteases in innate immunity.
Figures


References
-
- Alnemri ES, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. - PubMed
-
- Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265:75–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

