Diguanylate Cyclases AdrA and STM1987 Regulate Salmonella enterica Exopolysaccharide Production during Plant Colonization in an Environment-Dependent Manner
- PMID: 26655751
- PMCID: PMC4751842
- DOI: 10.1128/AEM.03475-15
Diguanylate Cyclases AdrA and STM1987 Regulate Salmonella enterica Exopolysaccharide Production during Plant Colonization in an Environment-Dependent Manner
Abstract
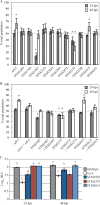
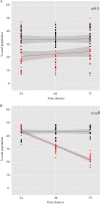
Increasing evidence indicates that despite exposure to harsh environmental stresses, Salmonella enterica successfully persists on plants, utilizing fresh produce as a vector to animal hosts. Among the important S. enterica plant colonization factors are those involved in biofilm formation. S. enterica biofilm formation is controlled by the signaling molecule cyclic di-GMP and represents a sessile lifestyle on surfaces that protects the bacterium from environmental factors. Thus, the transition from a motile, planktonic lifestyle to a sessile lifestyle may represent a vital step in bacterial success. This study examined the mechanisms of S. enterica plant colonization, including the role of diguanylate cyclases (DGCs) and phosphodiesterases (PDEs), the enzymes involved in cyclic di-GMP metabolism. We found that two biofilm components, cellulose and curli, are differentially required at distinct stages in root colonization and that the DGC STM1987 regulates cellulose production in this environment independent of AdrA, the DGC that controls the majority of in vitro cellulose production. In addition, we identified a new function for AdrA in the transcriptional regulation of colanic acid and demonstrated that adrA and colanic acid biosynthesis are associated with S. enterica desiccation tolerance on the leaf surface. Finally, two PDEs with known roles in motility, STM1344 and STM1697, had competitive defects in the phyllosphere, suggesting that regulation of motility is crucial for S. enterica survival in this niche. Our results indicate that specific conditions influence the contribution of individual DGCs and PDEs to bacterial success, perhaps reflective of differential responses to environmental stimuli.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Persistence of Salmonella enterica serovar Typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog Dis 1:27–35. doi: 10.1089/153531404772914437. - DOI - PubMed
-
- Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Fate of Salmonella enterica serovar Typhimurium on carrots and radishes grown in fields treated with contaminated manure composts or irrigation water. Appl Environ Microbiol 70:2497–2502. doi: 10.1128/AEM.70.4.2497-2502.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

