Evaluation of the Safety and Immunogenicity of a Candidate Pandemic Live Attenuated Influenza Vaccine (pLAIV) Against Influenza A(H7N9)
- PMID: 26655841
- PMCID: PMC4760421
- DOI: 10.1093/infdis/jiv526
Evaluation of the Safety and Immunogenicity of a Candidate Pandemic Live Attenuated Influenza Vaccine (pLAIV) Against Influenza A(H7N9)
Abstract
Background: We evaluated a candidate A/Anhui/2013(H7N9) pandemic live attenuated influenza vaccine (pLAIV) in healthy adults, and assessed the ability of 1 or 2 doses to induce immune memory.
Methods: Healthy subjects in 2 age groups (18-49 years and 50-70 years) with undetectable hemagglutination-inhibiting (HAI) antibody to H7N9 were enrolled. Younger subjects received either 1 or 2 intranasal doses of 10(7.0) fluorescent focus units of A/Anhui/1/2013 pLAIV, while older subjects received a single dose. All subjects received a single 30-µg dose of unadjuvanted, antigenically matched A/Shanghai2/2013(H7N9) pandemic inactivated influenza vaccine (pIIV) 12 weeks after their first dose of pLAIV.
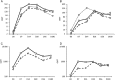
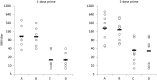
Results: Both vaccines were well tolerated. Serum HAI antibody responses were detected in 0 of 32 younger subjects and 1 of 17 older subjects after 1 dose of pLAIV and in 2 of 16 younger subjects after a second dose. Strong serum antibody responses were detected after a single subsequent dose of pIIV that was broadly reactive against H7 influenza viruses.
Conclusions: An A(H7N9) pLAIV candidate was safe in both age groups. Priming with pLAIV resulted in responses to subsequent pIIV that exceeded those seen in naive subjects in previous reports. The A(H7N9) pLAIV induces strong immune memory that can be demonstrated by exposure to subsequent antigenic challenge.
Clinical trials registration: NCT01995695 and NCT02274545.
Keywords: immune memory; live vaccine; pandemic influenza.
© The Author 2015. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- Gao H-N, Lu H-Z, Cao B et al. . Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013; 368:2277–85. - PubMed
-
- Ai J, Huang Y, Xu K et al. . Case-control study of risk factors for human infection with influenza A(H7N9) virus in Jiangsu province, China, 2013. Euro Surveillance 2013; 18:20510. - PubMed
-
- Mulligan MJ, Bernstein DI, Winokur PL et al. . Serological responses for an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1409–19. - PubMed
-
- Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-thasan N. A cell culture–derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Sci Transl Med 2014; 6:234ra55. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

