Tyro3 Modulates Mertk-Associated Retinal Degeneration
- PMID: 26656104
- PMCID: PMC4687644
- DOI: 10.1371/journal.pgen.1005723
Tyro3 Modulates Mertk-Associated Retinal Degeneration
Abstract
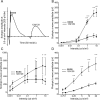
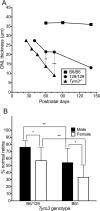
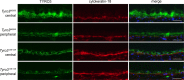
Inherited photoreceptor degenerations (IPDs) are the most genetically heterogeneous of Mendelian diseases. Many IPDs exhibit substantial phenotypic variability, but the basis is usually unknown. Mutations in MERTK cause recessive IPD phenotypes associated with the RP38 locus. We have identified a murine genetic modifier of Mertk-associated photoreceptor degeneration, the C57BL/6 (B6) allele of which acts as a suppressor. Photoreceptors degenerate rapidly in Mertk-deficient animals homozygous for the 129P2/Ola (129) modifier allele, whereas animals heterozygous for B6 and 129 modifier alleles exhibit an unusual intermixing of degenerating and preserved retinal regions, with females more severely affected than males. Mertk-deficient mice homozygous for the B6 modifier allele display degeneration only in the far periphery, even at 8 months of age, and have improved retinal function compared to animals homozygous for the 129 allele. We genetically mapped the modifier to an approximately 2-megabase critical interval that includes Tyro3, a paralog of Mertk. Tyro3 expression in the outer retina varies with modifier genotype in a manner characteristic of a cis-acting expression quantitative trait locus (eQTL), with the B6 allele conferring an approximately three-fold higher expression level. Loss of Tyro3 function accelerates the pace of photoreceptor degeneration in Mertk knockout mice, and TYRO3 protein is more abundant in the retinal pigment epithelium (RPE) adjacent to preserved central retinal regions of Mertk knockout mice homozygous for the B6 modifier allele. Endogenous human TYRO3 protein co-localizes with nascent photoreceptor outer segment (POS) phagosomes in a primary RPE cell culture assay, and expression of murine Tyro3 in cultured cells stimulates phagocytic ingestion of POS. Our findings demonstrate that Tyro3 gene dosage modulates Mertk-associated retinal degeneration, provide strong evidence for a direct role for TYRO3 in RPE phagocytosis, and suggest that an eQTL can modify a recessive IPD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Kajiwara K, Berson EL, Dryja TP (1994) Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264: 1604–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

