Lysozyme Photochemistry as a Function of Temperature. The Protective Effect of Nanoparticles on Lysozyme Photostability
- PMID: 26656259
- PMCID: PMC4682814
- DOI: 10.1371/journal.pone.0144454
Lysozyme Photochemistry as a Function of Temperature. The Protective Effect of Nanoparticles on Lysozyme Photostability
Abstract
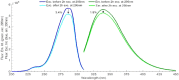
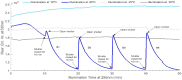
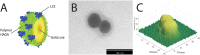
The presence of aromatic residues and their close spatial proximity to disulphide bridges makes hen egg white lysozyme labile to UV excitation. UVB induced photo-oxidation of tryptophan and tyrosine residues leads to photochemical products, such as, kynurenine, N-formylkynurenine and dityrosine and to the disruption of disulphide bridges in proteins. We here report that lysozyme UV induced photochemistry is modulated by temperature, excitation power, illumination time, excitation wavelength and by the presence of plasmonic quencher surfaces, such as gold, and by the presence of natural fluorescence quenchers, such as hyaluronic acid and oleic acid. We show evidence that the photo-oxidation effects triggered by 295 nm at 20°C are reversible and non-reversible at 10°C, 25°C and 30°C. This paper provides evidence that the 295 nm damage threshold of lysozyme lies between 0.1 μW and 0.3 μW. Protein conformational changes induced by temperature and UV light have been detected upon monitoring changes in the fluorescence emission spectra of lysozyme tryptophan residues and SYPRO® Orange. Lysozyme has been conjugated onto gold nanoparticles, coated with hyaluronic acid and oleic acid (HAOA). Steady state and time resolved fluorescence studies of free and conjugated lysozyme onto HAOA gold nanoparticles reveals that the presence of the polymer decreased the rate of the observed photochemical reactions and induced a preference for short fluorescence decay lifetimes. Size and surface charge of the HAOA gold nanoparticles have been determined by dynamic light scattering and zeta potential measurements. TEM analysis of the particles confirms the presence of a gold core surrounded by a HAOA matrix. We conclude that HAOA gold nanoparticles may efficiently protect lysozyme from the photochemical effects of UVB light and this nanocarrier could be potentially applied to other proteins with clinical relevance. In addition, this study confirms that the temperature plays a critical role in the photochemical pathways a protein enters upon UV excitation.
Conflict of interest statement
Figures





Similar articles
-
EGF Functionalized Polymer-Coated Gold Nanoparticles Promote EGF Photostability and EGFR Internalization for Photothermal Therapy.PLoS One. 2016 Oct 27;11(10):e0165419. doi: 10.1371/journal.pone.0165419. eCollection 2016. PLoS One. 2016. PMID: 27788212 Free PMC article.
-
Flash photolysis of cutinase: identification and decay kinetics of transient intermediates formed upon UV excitation of aromatic residues.Biophys J. 2009 Jul 8;97(1):211-26. doi: 10.1016/j.bpj.2009.01.065. Biophys J. 2009. PMID: 19580759 Free PMC article.
-
Optical characterization and tunable antibacterial properties of gold nanoparticles with common proteins.Anal Biochem. 2021 Jan 1;612:113975. doi: 10.1016/j.ab.2020.113975. Epub 2020 Sep 20. Anal Biochem. 2021. PMID: 32966803
-
Photochemistry of proteins: a review.Curr Eye Res. 1984 Jan;3(1):137-44. doi: 10.3109/02713688408997195. Curr Eye Res. 1984. PMID: 6360538 Review.
-
Photo-induced protein modifications: a range of biological consequences and applications.Biophys Rev. 2023 Jul 1;15(4):569-576. doi: 10.1007/s12551-023-01081-6. eCollection 2023 Aug. Biophys Rev. 2023. PMID: 37681095 Free PMC article. Review.
Cited by
-
EGF Functionalized Polymer-Coated Gold Nanoparticles Promote EGF Photostability and EGFR Internalization for Photothermal Therapy.PLoS One. 2016 Oct 27;11(10):e0165419. doi: 10.1371/journal.pone.0165419. eCollection 2016. PLoS One. 2016. PMID: 27788212 Free PMC article.
-
Sound-mediated nucleation and growth of amyloid fibrils.Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2315510121. doi: 10.1073/pnas.2315510121. Epub 2024 Aug 12. Proc Natl Acad Sci U S A. 2024. PMID: 39133851 Free PMC article.
-
Selective Modification of Tryptophan Residues in Peptides and Proteins Using a Biomimetic Electron Transfer Process.J Am Chem Soc. 2020 May 20;142(20):9112-9118. doi: 10.1021/jacs.0c03039. Epub 2020 May 5. J Am Chem Soc. 2020. PMID: 32348670 Free PMC article.
-
Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems.Pharmaceutics. 2019 Jan 8;11(1):22. doi: 10.3390/pharmaceutics11010022. Pharmaceutics. 2019. PMID: 30625999 Free PMC article. Review.
-
UV cross-linked polyvinylpyrrolidone electrospun fibres as antibacterial surfaces.Sci Technol Adv Mater. 2019 Sep 17;20(1):979-991. doi: 10.1080/14686996.2019.1667737. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 31692919 Free PMC article.
References
-
- Lakowicz JR. Principles of fluorescence spectroscopy. 2nd ed New York: Academic/ Plenum Publishers; 1999.
-
- Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305(3): 761–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

