A Multicenter Reference Intervals Study for Specific Proteins in China
- PMID: 26656356
- PMCID: PMC5008501
- DOI: 10.1097/MD.0000000000002211
A Multicenter Reference Intervals Study for Specific Proteins in China
Abstract
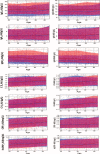
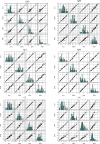
A multicenter study conducted in healthy population of 6 cities from the 4 corners and central China for 7 serum-specific proteins to identify the sources of variation and establish the reference intervals on 2 automation platforms.A total of 3148 subjects aged 19 to 64 years old were enrolled in this study to ensure at least 120 participants in each 10-year age group and each city. The majority of samples were transported to central laboratory and measured on both Beckman AU5800 and Immage 800 analytical systems. Three-level nested ANOVA, multiple regression analysis, and the scatter plot were used to explore the variations from sex, age, region, BMI, cigarette smoking, and so on. The latent abnormal value exclusion (LAVE) method was applied at the time of computing RIs as a method for secondary exclusion.Regionality was not observed in any of the immunoassay in China. Variations for sex were significant for IgM among the immune analytes. For CRP and hsCRP results with turbidimetry method (Beckman Coulter AU5800) were lower than the nephelometry method (Beckman Immage). The LAVE method did not affect the RIs computed for the majority of analytes except C4, CRP, and hsCRP. In the scatter plot at the age of 45 years old C3, C4, and IgM reached an inflection point, accordingly RIs were separated by the age group.With the lack of regional differences and the well-standardized status of test results, the RIs of C3, IgG, IgA, IgM derived from this nationwide study can be used for the entire Chinese population. C4, CRP, and hsCRP were affected by different platforms and gender was a significant source of variation for IgM, so they had separated RIs.
Conflict of interest statement
The authors have no conflicts of interest to disclose. Research funding played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Figures

Similar articles
-
Derivation of sex and age-specific reference intervals for clinical chemistry analytes in healthy Ghanaian adults.Clin Chem Lab Med. 2022 Jul 4;60(9):1426-1439. doi: 10.1515/cclm-2022-0293. Print 2022 Aug 26. Clin Chem Lab Med. 2022. PMID: 35786502
-
A multicenter nationwide reference intervals study for common biochemical analytes in Turkey using Abbott analyzers.Clin Chem Lab Med. 2014 Dec;52(12):1823-33. doi: 10.1515/cclm-2014-0228. Clin Chem Lab Med. 2014. PMID: 25153598
-
A nationwide multicentre study in Turkey for establishing reference intervals of haematological parameters with novel use of a panel of whole blood.Biochem Med (Zagreb). 2017 Jun 15;27(2):350-377. doi: 10.11613/BM.2017.038. Biochem Med (Zagreb). 2017. PMID: 28694726 Free PMC article.
-
Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative.Clin Chem Lab Med. 2018 Nov 27;56(12):2093-2103. doi: 10.1515/cclm-2018-0152. Clin Chem Lab Med. 2018. PMID: 30074895 Clinical Trial.
-
[Reference Intervals of Standard Test Items in Ningen Dock Examination].Rinsho Byori. 2016 Mar;64(3):270-7. Rinsho Byori. 2016. PMID: 27363219 Review. Japanese.
Cited by
-
Prevalence of Neutralizing Antibodies Against AAV Serotypes 2 and 9 in Healthy Participants from Multiple Centers Across China and Patients with DMD/BMD.Hum Gene Ther. 2024 Dec;35(23-24):969-977. doi: 10.1089/hum.2024.079. Epub 2024 Nov 28. Hum Gene Ther. 2024. PMID: 39607725
-
The indirect method in the establishment of reference intervals for complement 3 and complement 4: A retrospective study.J Taibah Univ Med Sci. 2021 Dec 1;17(3):353-361. doi: 10.1016/j.jtumed.2021.10.011. eCollection 2022 Jun. J Taibah Univ Med Sci. 2021. PMID: 35722234 Free PMC article.
-
Identification and functional analysis of GCK gene mutations in 12 Chinese families with hyperglycemia.J Diabetes Investig. 2019 Jul;10(4):963-971. doi: 10.1111/jdi.13001. Epub 2019 Feb 1. J Diabetes Investig. 2019. PMID: 30592380 Free PMC article.
References
-
- CLSI, C.A.L.S. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline third edition. Third edition. CLSI document C28-A3. 2010.
-
- Ye YF, W.Y.S.Z. National guide to Clinical Laboratory Procedures (third edition) [Chinese.]. Southeast University Press. Southeast University Press. 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

