A Multicenter Reference Intervals Study for Specific Proteins in China
- PMID: 26656356
- PMCID: PMC5008501
- DOI: 10.1097/MD.0000000000002211
A Multicenter Reference Intervals Study for Specific Proteins in China
Abstract
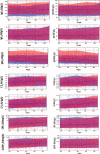
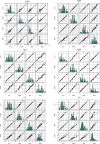
A multicenter study conducted in healthy population of 6 cities from the 4 corners and central China for 7 serum-specific proteins to identify the sources of variation and establish the reference intervals on 2 automation platforms.A total of 3148 subjects aged 19 to 64 years old were enrolled in this study to ensure at least 120 participants in each 10-year age group and each city. The majority of samples were transported to central laboratory and measured on both Beckman AU5800 and Immage 800 analytical systems. Three-level nested ANOVA, multiple regression analysis, and the scatter plot were used to explore the variations from sex, age, region, BMI, cigarette smoking, and so on. The latent abnormal value exclusion (LAVE) method was applied at the time of computing RIs as a method for secondary exclusion.Regionality was not observed in any of the immunoassay in China. Variations for sex were significant for IgM among the immune analytes. For CRP and hsCRP results with turbidimetry method (Beckman Coulter AU5800) were lower than the nephelometry method (Beckman Immage). The LAVE method did not affect the RIs computed for the majority of analytes except C4, CRP, and hsCRP. In the scatter plot at the age of 45 years old C3, C4, and IgM reached an inflection point, accordingly RIs were separated by the age group.With the lack of regional differences and the well-standardized status of test results, the RIs of C3, IgG, IgA, IgM derived from this nationwide study can be used for the entire Chinese population. C4, CRP, and hsCRP were affected by different platforms and gender was a significant source of variation for IgM, so they had separated RIs.
Conflict of interest statement
The authors have no conflicts of interest to disclose. Research funding played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Figures

References
-
- CLSI, C.A.L.S. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline third edition. Third edition. CLSI document C28-A3. 2010.
-
- Ye YF, W.Y.S.Z. National guide to Clinical Laboratory Procedures (third edition) [Chinese.]. Southeast University Press. Southeast University Press. 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

